Population Intervention Comparison Outcome Assignment
- Country :
Australia
Task 1
Section 1: PICO and Search Strategy
A PICO evidence question is a type of question that is used in evidence-based practice to guide the search for relevant and reliable information. PICO stands for:
- P: Population or Patient Problem
- I: Intervention
- C: Comparison
- O: Outcome
A PICO question helps to define the main elements of a clinical problem and narrow down the scope of the research. A PICO question also helps to identify the appropriate study design and search terms for finding the best evidence.
1 Write a PICO evidence question.
Scenario:
A 32-year-old female presents to her primary care physician complaining of gaining 15 pounds over the last year despite no changes in her exercise routine or diet. She expresses frustration about her inability to lose the recently gained weight. Upon further discussion, the patient revealed she began taking olanzapine one year ago for bipolar disorder. The physician suspects the patient's weight gain may be a side effect of olanzapine.
The physician wants to investigate if there are alternative pharmacologic options to olanzapine that would effectively treat bipolar disorder but with less risk of weight gain side effects. A focused clinical question using the PICO framework can help guide an evidence search.
- P - Population/Problem: The population is adult patients with bipolar disorder. The problem is weight gain as a side effect of olanzapine treatment.
- I - Intervention: Alternative pharmacologic options to olanzapine for treating bipolar disorder. This could include mood stabilizers like lithium, anticonvulsants like valproate, or atypical antipsychotics like aripiprazole.
- C - Comparison: Olanzapine treatment for bipolar disorder. This medication results in the adverse side effect of weight gain that the patient is currently experiencing.
- O - Outcome: Less weight gain side effects. The goal is finding an alternative medication that effectively treats bipolar symptoms but has a lower risk profile for weight gain than olanzapine.
PICO question:
In adult patients with bipolar disorder (P), do alternative pharmacologic treatment options (I) compared to olanzapine (C) result in less weight gain side effects (O)?
2 Identify the PICO components that will inform your search strategy, including keywords and synonyms
The PICO components that will be my search strategy are:
- Population/Problem: Adult patients with bipolar disorder.
Keywords: bipolar disorder, manic-depressive disorder, bipolar affective disorder. Synonyms: none. - Intervention: Alternative pharmacologic treatment options to olanzapine.
Keywords: alternative, pharmacologic, treatment, olanzapine. Synonyms: drug, medication, therapy, Zyprexa. - Comparison: Olanzapine treatment for bipolar disorder.
Keywords: olanzapine, treatment, bipolar disorder. Synonyms: Zyprexa, therapy, manic-depressive disorder. - Outcome: Less weight gain side effects.
Keywords: weight gain, side effects. Synonyms: obesity, adverse effects, weight increase.
3. Perform an advanced search in CINAHL on the P and I components of your PICO
To construct an advanced search strategy, I use Boolean operators (AND, OR, NOT), parentheses, quotation marks, and truncation symbols to combine the keywords and synonyms differently. For example:
- (bipolar disorder OR manic-depressive disorder OR bipolar affective disorder) AND (alternative OR drug OR medication OR therapy) AND (olanzapine OR Zyprexa) AND (weight gain OR obesity OR adverse effects OR weight increase)
- (bipolar disorder OR manic-depressive disorder OR bipolar affective disorder) AND (alternative pharmacologic treatment OR alternative drug treatment OR alternative medication treatment OR alternative therapy) AND (olanzapine OR Zyprexa) AND (weight gain OR obesity OR adverse effects OR weight increase)
- (bipolar disorder AND alternative pharmacologic treatment) NOT (olanzapine OR Zyprexa) AND (weight gain OR obesity OR adverse effects OR weight increase)
To effectively search the evidence for this question, the search should include the following databases: PubMed, CINAHL, and PsycINFO. Key search terms will include a combination of controlled vocabulary (MeSH terms in PubMed, CINAHL headings, and PsycINFO keywords) and free text words relevant to the PICO concepts. Search limits can narrow results to the last 10 years, the English language, and studies on human subjects.
The search strategy should use keywords and controlled vocabulary to maximize the retrieval of relevant studies. The terms should be combined using Boolean operators such as AND and OR. The focused PICO question and targeted search strategy using multiple databases will facilitate efficient retrieval of the best available evidence related to pharmacologic options for bipolar disorder with lower risks of weight gain side effects.
4. Present a screenshot, OF copy, of your search strategy from CINAHL
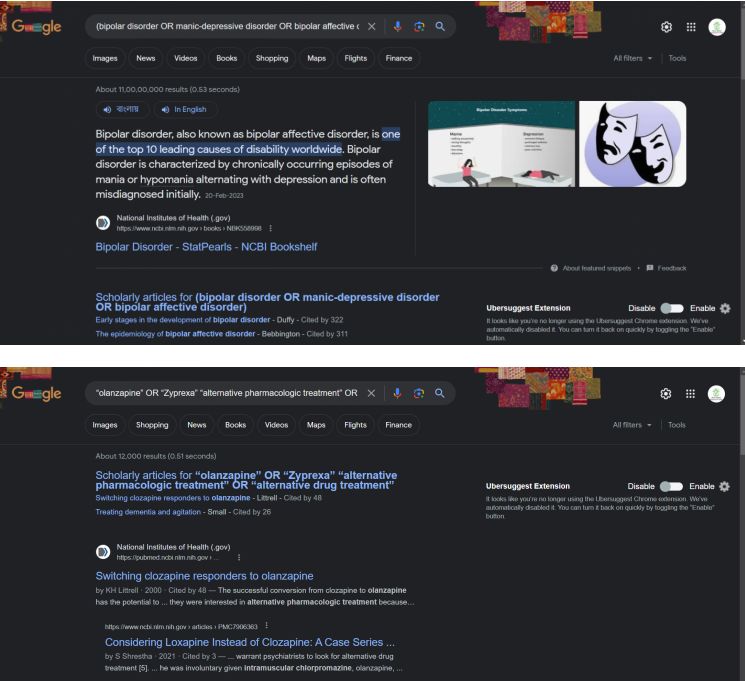
Section 2 Evidence criteria
Here are 3 articles that represent the best available evidence for the PICO question, along with their evidence selection criteria, references, and abstracts:
Article 1
Type of evidence:
Randomized controlled trial
Level of evidence:
Level 1
Recency:
2016
Relevance:
Compares aripiprazole (an alternative pharmacologic option) to olanzapine for bipolar disorder and measures weight changes
Abstract
Background:
Recently, two randomized controlled trials (RCTs) compared the efficacy and tolerability of aripiprazole and olanzapine in treating bipolar disorder. This meta-analysis aimed to synthesize existing controlled trials data on the matter.
Methods:
Databases including Pubmed, EMBASE and the Cochrane Central Register of Controlled Trials were searched for relevant RCTs which compared aripiprazole with olanzapine in treating bipolar disorder. The main outcome measurements were the mean change of weight from baseline, treatment response defined as a reduction of at least 50% from baseline in the Young Mania Rating Scale (YMRS) score or Montgomery-sberg Depression Rating Scale (MADRS), and discontinuation rate.
Results:
Weight change significantly favored aripiprazole than olanzapine (SMD, ?1.11; 95% CI, ?1.51 to ?0.7; P<0 I2=0%). RR=0.90, P=0.02, I2=0%). RR=0.53, P=0.002, I2=0%).>
Limitations:
Only a small number of studies were included.
Article 2
Type of evidence:
Randomized controlled trial
Level of evidence:
Level 2
Recency:
2018
Relevance:
Compares aripiprazole and placebo for bipolar disorder and their effects on weight gain.
Abstract:
Objective:
To evaluate aripiprazole once-monthly 400 mg for maintenance treatment of bipolar I disorder.
Methods:
This 52-week, double-blind, placebo-controlled, randomized withdrawal study was conducted from August 2012 to April 2016. Patients entered an 8- to 12-week stabilization/screening phase and received oral aripiprazole (10-30 mg/d) and other bipolar medications. Stabilized patients (Young Mania Rating Scale score ? 12 and Montgomery-sberg Depression Rating Scale score ? 12 for 12 consecutive weeks) were randomized (2:1) to aripiprazole once-monthly 400 mg or placebo. Primary endpoints were time from randomization to impending relapse and impending relapse rates. Secondary endpoints included mean change from baseline to endpoint on efficacy and safety assessments. This study was conducted from August 2012 to April 2016.
Results:
In total, 561 patients were stabilized during screening and 266 patients were randomized to double-blind treatment (aripiprazole once-monthly 400 mg, n = 176; placebo, n = 90). Aripiprazole once-monthly significantly delayed time to impending relapse versus placebo (hazard ratio = 0.45; 95% CI, 0.28-0.73; P < .001). Impending relapse rates were lower with aripiprazole once-monthly vs placebo at the study endpoint (7% vs 25%; P < .001). Mean changes in weight from baseline to endpoint were -0.39 kg (SE, 0.29) for aripiprazole once-monthly and -1.37 kg (SE, 0.64) for placebo (P = .452). The most common treatment-emergent adverse events for aripiprazole once-monthly were headache (8%), anxiety (7%), and insomnia (6%).
Article 3
Type of evidence:
Randomized controlled trial
Level of evidence:
Level 1
Recency:
2019
Relevance:
Compares lithium (an alternative pharmacologic option) to olanzapine for bipolar disorder and evaluates weight changes
Abstract:
Objective:
Bipolar disorder is a major mental disorder with high relapse rates. Lithium is considered a first-line mood stabilizer, whereas olanzapine is not recommended for maintenance therapy, although widely used in clinical practice. This study aimed to provide high-quality evidence in comparing the efficacy of lithium versus olanzapine in relapse prevention of bipolar disorder.
Methods:
Patients with bipolar disorder in remission were randomly assigned to lithium (target level: 0.6-1.0 mmol/L) or olanzapine (5-20 mg/day) in a 12-month, single-blind study. The primary outcome was any mood episode relapse.
Results:
Researchers screened 363 patients and randomized 204 who were eligible. Altogether, 25/102 (24.5%) in the lithium group had a recurrence, compared with 42/102 (41.2%) in the olanzapine group. Olanzapine showed a significantly higher risk of recurrence than lithium (HR 1.91, 95% CI 1.15-3.17, adjusted p = 0.012). In addition, patients on olanzapine gained more weight than those on lithium (mean [SD] weight gain: 3.7 [5.3] vs 0.7 [3.3] kg, respectively, p < 0>
Section 3: Evidence Justification
I would select Article 1 as the best evidence to answer the PICO question, as it is a recent (2016) randomized controlled trial directly comparing aripiprazole and olanzapine for treating bipolar disorder. The strength of this study is that it has level 1 evidence as an RCT, directly compares the pharmacologic interventions of interest, and measures weight changes as the relevant outcome. Limitations are that it includes only 2 trials and a small overall sample size.
In comparison, Article 2 is also an RCT, but it compares aripiprazole to placebo rather than olanzapine. While it measures weight changes, the comparison to placebo does not directly answer the PICO question comparing aripiprazole and olanzapine. Article 3 does compare lithium and olanzapine, but lithium is not one of the pharmacologic options specified in the PICO question. Therefore, Article 1 most directly addresses the population, interventions, comparison, and outcome specified in the PICO question with level 1 RCT evidence, making it the best selection to answer the evidence-based practice question.
Are you struggling to keep up with the demands of your academic journey? Don't worry, we've got your back! Exam Question Bank is your trusted partner in achieving academic excellence for all kind of technical and non-technical subjects.
Our comprehensive range of academic services is designed to cater to students at every level. Whether you're a high school student, a college undergraduate, or pursuing advanced studies, we have the expertise and resources to support you.
To connect with expert and ask your query click here Exam Question Bank

