CHEE3735 Mass Transfer Processes Assignment
- Subject Code :
CHEE3735
- Country :
Australia
Assignment Requirements
To obtain full marks, assignments must include:
- Clear statement of all assumptions used (these should be verified where possible).
- Diagram showing process and system boundaries, summarising information from the question.
- Clear definition of variables.
- Citation of source of equations and solution method used (if not directly from the lecture notes).
- Citation of sources of all material property data used in sufficient detail so that the reader can also find the same data i.e. dont just say steam tables. Quote the actual source website or textbook.
- Units must be shown clearly with all numbers. In equations you should include units and show how they cancel to give the units of the calculated result. An answer without its units is meaningless!
- You should also include a complete set of sample calculations. You need to clearly show (i) what equations you have used and (ii) what numerical values you have substituted (together with their units). For example, to demonstrate how Reynolds number is calculated, you should detail the full calculation as: Re = ?uD/? = (1000 kg/m3 )(1 m/s)(1 m)/(0.001 Pa s) = 106. This level of detail is necessary because in enables the marker to clearly see what youve done and therefore to still be able to award partial marks even if your final answer is wrong.
- When a solution involves an iterative method of repeated calculations, then you should include a summary table showing the results of all calculation steps as well as sample calculations for one full cycle of the iterative method. NOTE: A printout of a spreadsheet may be a useful summary of all your results, but it does NOT constitute sample calculations because the marker cant see what the equations or substituted values are. The spreadsheet table should be supported with sample calculations for one complete cycle/iteration of calculations.
- You should always discuss your results and whenever possible check they are reasonable, even if not explicitly asked to.
Purpose: To encourage students to revise course content in a systematic and timely manner.
- Summaries may be handwritten. Keep original and submit scanned pdf copy on Canvas.
- Feel free to use diagrams, sample calculations, mind maps, etc.
- One (1) page is enough, but it can be longer.
Over the course of the semester a cumulative total of 14 days (or part days) of late submissions is permitted without penalty. Once that quota is exceeded, summaries submitted late will receive zero marks.
This assignment concerns the distillation of a feed mixture of 60 mol percent lauric acid (Component A) and 40 mol percent myristic acid (Component B). These acids are obtained from various plant oils and find use in a range of skin products. The distillation is to be carried out at a pressure of 7.5 mm Hg (= 1000 Pa). The vapour pressures of the pure components are given by the equations:
ln(Pvap,A) = 20.8375 2210/T 1 715 400/T2
ln(Pvap,B) = 19.9356 1467/T 2 072 300/T2
where vapour pressure Pvap is in Pa and temperature T is in K.
- Assuming Raoults law is valid, determine and plot the vapour?liquid equilibrium curve for this mixture (at 1000 Pa). Calculate at least 10 points on the curve.
- If this feed enters a flash drum where 60 mol % is vapourised, then determine the concentrations of the vapour and liquid products, the temperatures of these two streams, and the percentage recovery of lauric acid to the vapour stream.
- If instead the feed is distilled in a batch still until 60 mol % has been recovered as distillate product, determine the concentrations of the distillate product and residue remaining in the still, the temperature of the remaining contents of the still, and the recovery of lauric acid to the distillate product.If the feed enters as a saturated liquid at its boiling point into a continuous counter?current distillation column, operated to give an overheat product with 90 mol % lauric acid and a bottoms product with 15 mol % lauric acid, then:
- Determine the flows of overhead distillate product D and bottoms product W per 100 mol of feed. Determine also the temperatures of both streams and the percentage recovery of lauric acid to the distillate product.
- Comment on the pros and cons of the three types of distillation (Parts 2, 3 and 4).
- Determine the minimum number of stages Nmin that could be used for this separation (i.e. with an infinite reflux).
- Determine the minimum reflux ratio Rmin that could be used for thisseparation (i.e. with an infinite number of stages).
- If an actual reflux ratio of R = 1 is used, then determine (i) the number of theoretical stages required, (ii) the location of the feed tray, and (iii) the flows of vapour and liquid streams in both the rectifying and stripping sections of the column (per 100 mol of feed).
- Determine the reflux ratio R required (to one decimal place accuracy) that would result in exactly five (5) theoretical stages being needed (you may need to use trial and error to find the solution).
- If the Murphree stage efficiency was 70 %, then determine the number of real trays required if a reflux ratio of R = 1 were used.
QUESTION 1: Single Film One?way Diffusion (20 %)
The diffusion coefficients of vapours can be calculated by measuring the evaporation rate of a liquid from a vertical tube. Based on the published diffusivity in air of 0.071 cm2 /s, estimate the expected rate of evaporation (mm/h liquid level drop) of n?heptane (C7H16) from a 2 mm diameter tube at 21 ?C and 1 atm when the liquid level is 10 mm from the top of the tube. At this temperature the vapour pressure of n?heptane is 0.050 atm and the liquid density is 0.66 g/cm3. Discuss whether varying the tube diameter would give any benefit.
QUESTION 2: Two?Phase Mass Transfer (30 %)
Consider the equi?molar counter diffusion of component A from a liquid phase into a gas phase. The equilibrium data indicate that at low concentrations yA = 12.0xA. If the bulk concentrations in the gas and liquid phases are yA = 0.05 and xA = 0.01 respectively, and ky = 8.0?10?6 kmol/(m2 s) and kx = 6.67?10?5 kmol/(m2 s).
Determine the interface concentrations in both phases and the rate of mass transfer. You must use two methods, the first being expressions based on the local mass transfer coefficients, and the second based on the use of an overall gas phase mass transfer coefficient. Based on the second method, calculate the percentage (%) of the resistance to mass transfer in each of the two phases. Provide a y versus x graph showing the equilibrium curve and plot the relevant data.
QUESTION 3: Rising Oxygen Bubble Mass Transfer (50 %)
Air is often bubbled through liquids to restore the oxygen concentration after it has been consumed by various biological or chemical processes. Estimate the fraction of oxygen absorbed from a 4.0 mm diameter air bubble as it rises a distance of 1 m through water at 20 ?C which contains no dissolved oxygen. Assume free circulation of the air inside the bubble, and so Higbie penetration theory applies. You will need to make many other assumptions and look up various material property data in order to solve this question. Make sure you clearly state your assumptions and where possible state whether each assumption causes an under or over?estimate in your final answer (this is worth significant marks in this question). State clearly also the sources of any data you use.
NOTE: Gas bubbles do not rise at the same velocity as solid spheres. Look up some correlations!
Absorption Column
Air containing 5 mol.?nzene (C6H6) is to be cleaned in a counter?current column. It is required that 90 % of the benzene be removed from the air before it is released. 27.3 lbmol/h of a non?volatile wash oil (molecular weight 230, viscosity 0.004 Pa?s, specific gravity 0.9) is used to absorb the benzene. The wash oil enters at the top of the column already containing 0.2 wt.?nzene. The inlet feed gas and the column operate at uniform conditions of 80 ?F and 1 atm. The incoming air?flowrate is 40,000 ft3/h at a superficial mass velocity of 800 lb/(ft2 h). You may assume that the benzene?oil mixture is an ideal mixture and that the vapour pressure of benzene at 80 ?F is 106 mm Hg.
- Convert the inlet oil concentration to a mole fraction.
- Express the inlet gas volumetric flowrate to a mass (lb/h) and a molar (lbmol/h) flowrate.
- Determine the target concentration of benzene in the exit gas stream.
- Perform a material balance around the column to determine the exit concentration of benzene in the wash oil. Is this a lean or rich phase system?
- Use the benzene vapour pressure information to derive the equilibrium relationship (i.e. y* = f(x)) for this system (assume this is an ideal mixture so Raoults law applies).
- Determine the minimum liquid oil flowrate that could be used to achieve the required separation (i.e. with an infinite column).
- Determine the number of stages needed (for a wash oil rate of 27.3 lbmol/h).
- Determine the cross?sectional area of the column (use the superficial mass velocity).
- Determine the flooding limit liquid flowrate.
- Calculate the number of transfer units required (for a wash oil rate of 27.3 lbmol/h).
- Use a correlation to estimate the height of a transfer unit for this system if it can be assumed that the gas?phase resistance dominates.
- Determine the height of packing required.
QUESTION 1: Extraction (50 %)
It is desired to recover 86.5 % of the acetone from a stream containing 50 wt.?etone and 50 wt.% chloroform. Pure water is to be used as the solvent, at a solvent mass rate 1.5 times the feed mass rate. For a counter?current staged process, determine:
- The composition and flowrates of the final extract and raffinate streams.
- The number of stages required.
- Determine the exit concentrations and amounts of the extract and raffinate.
- Determine the recovery of acetone from the feed into the final extract.
- Based on your results in parts a?d, comment on the pros and cons of batch versus staged counter?current separations.
Data: Equilibrium data for the water?acetone?chloroform system is presented in the table below.
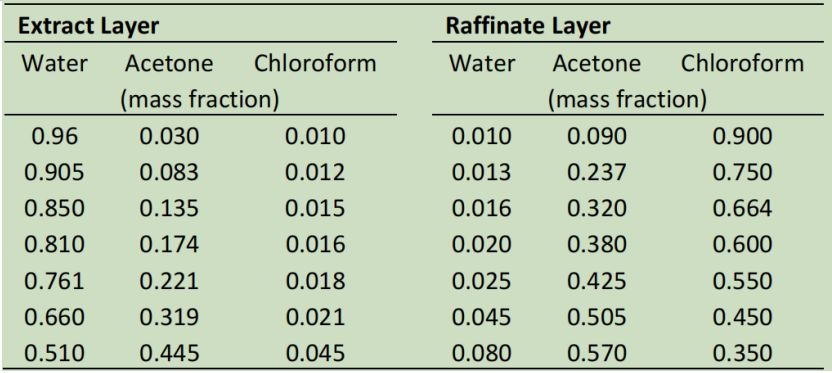
Each row gives the composition of the two layers in equilibrium.
QUESTION 2: Leaching (50 %)
A feed contains 82 % inert solids and 18 % soluble material. Both the inert and soluble material have a density of 2700 kg/m3. Using a counter?current staged process it is desired to recover 98 % of the soluble material from the feed into the final overflow product which is to have a concentration of 100 kg/m3 (solute mass per solution volume). The solvent density is 900 kg/m3. In order for it to be pumped from stage to stage, the process requires 200 volumes of liquid solution per 100 volumes of solid in the underflow stream. Determine:
- The concentrations of the final exit overflow and underflow streams; and
- The number of stages required.
If your grade in this optional assessment is higher than your final exam grade then this assignment will be worth 8.5 % of your final course grade (and your final exam weighting will be reduced by 8.5 % from 28.5 % to 20 %). Students must apply in written for approval of their topic choice at least four (4) weeks before the assignment due date.
TOPIC: How a Separation Process in CHEE3735 is used in a Specific Industry
Pick a current employer that you might want to apply for vacation work with at the end of this year or for a job with when you graduate, whose business involves using at least one of the mass?transfer separation unit operations learnt about in this course (i.e. distillation, absorption/stripping, liquid? liquid extraction, solid leaching, gas adsorption)*. Write a short report on how they use this unit operation which should include:
- An introduction with a flowsheet diagram of the entire process that involves the chosen separation unit operation, with a brief explanation of how the whole system works (i.e. including reactors, pumps and other unit operations in the flowsheet). (20 %)
- More detailed explanation of the chosen mass?transfer separation device and how it operates e.g. potentially including but not limited to specification of flowrates, compositions, phase, temperatures and pressures of all inlet and outlet streams, and possibly internal streams also (where applicable), diagram/photos of the device showing dimensions, fabrication materials, and relevant internal details, detailed discussion of how the separation process works, possibly including mass and energy balances, key mass transfer equations, and calculations of stages or number of transfer units (where applicable), (iv) description of how it is monitored and controlled, (v) what (if any) routine maintenance and servicing is required and how it is performed, (vi) discussion of any safety issues and/or operational problems that can arise and how they are dealt with, and (vii) discuss any other aspects of the operation that you think are of interest, especially aspects that are not covered in the CHEE3735 course notes. (70 %)
- 10 % of marks will be awarded for the quality of written English and formatting. TOPIC PRE?APPROVAL: I dont want large numbers of students to all contact and overwhelm a single local company and thus cause a strain on its relationship with the university. So before you start this assessment, you MUST apply in writing to me (email is fine), specifying the name of the company and the nature of the separation process involved and WAIT for my written approval. If I have already approved other students to research a process at that company then you may be refused. Approval MUST be applied for at least four (4) weeks prior to the assignment due date. You will not be penalised if you later decide not to do this optional assessment, but the sooner you let me know you are dropping it, the sooner I can potentially allow another student to approach that employer.
*If you have an interest in a company that uses some separation process not included in the list above, then I may be willing to allow it provided the process involves mass?transfer (i.e. diffusion) of some form (i.e. not just a physical separation like filtering or density separation). Please apply in writing to me and WAIT for my approval before you approach the company. FORMAT: Maximum of five (5) single?sided A4 pages of minimum 11 point, Calibri font, double?line spaced written text with minimum 2 cm margins. This page limit does NOT include the university assessment item cover sheet, title page, table of contents, reference list (APA 7 style), nomenclaturelist, or diagrams and tables. There is no need for an abstract, executive summary or conclusions. You should use appropriate section and sub?sections divisions with numbered headings. Put any diagrams/tables at the end of the document where they should be numbered and captioned and referred to by their number in the written report. You may include as many diagrams as you need and make them as large as you like in order to make it clear what you are talking about however, this freedom should not be abused as a way of squeezing in extra written text into your report. It is intended that tables would only be used for summarising stream property data, not for lists of written points.

