Organic Chemistry : compound synthesis Assessment
- Country :
Australia
General Forma? ng:
ALL text should be Arial 12pt. font, double-spaced. Document margins should be 1 on all sides. All chemical structures MUST be drawn using ChemDraw using Document Se? ngs ACS Document 1996. Text limits where described below apply, however, there is no page limit or limit on the number of fifigures within this document. You will be graded on content, by giving you word limits you are required to make every word of your manuscript count!
Final Paper Outline:
1. Background/Introduction (500 word text limit, no limit on text in Figures, 5% of grade)
Describe any unique properties of your target compound. This could include a brief discussion of the biological activity of your target, where it was isolated (natural product) or how it was discovered, unique aspects of the chemical structure and any additional information that you find significant.
2. Retrosynthesis (500 word text limit, no limit on text in Figures, 10% of grade)
Draw a figure in ChemDraw showing the complete retrosynthesis for your molecule. This figure should highlight in retrosynthetic form all of the key bonds that you targeted for assembly in your synthesis. In the text briefly describe why you chose to synthesis your molecule using the route shown. If alternate viable routes exist you should show this alternate route by including a second retrosynthesis drawn using ChemDraw. In this case your text should succinctly describe the key features that make your route better then the alternative approaches.
. Forward Synthesis Summary Page (NO TEXT, One page limit, no limit on text in Figures, 5% of grade)
Draw a figure using ChemDraw that separately shows EVERY STEP of the synthesis of your target compound. All reagents and reaction information should be included in this synthesis and every intermediate product should be drawn including all structural and stereochemical information. You should not include any mechanistic information into this summary page, your discussion of key reaction mechanisms will occur in the next section.
Forward Synthesis (1000 word text limit, no limit on text in Figures, 20% of grade)
Provide a brief description for EVERY STEP of your synthesis. You will be required to include a description of the mechanism and draw important mechanistic intermediates/transition states using ChemDraw for all key steps in your synthesis. You do not need to provide this level of detail for routine reactions, I will assist you in defining the key steps in your synthesis that require this more detailed analysis and discussion.
Supporting Information (NO TEXT, 5% of grade)
Provide precedent for EVERY STEP of your synthesis. For each chemical reaction you propose you should show the proposed reaction and immediately below this reaction include the Precedent for this reaction. Precedent is required for every step of your synthesis and should be in the form of a screen-shot of the results listing from a SciFinder search. These screenshot images should be appropriately cropped to include ONLY one reaction result, reaction information and reference information from SciFinder. [NOTE: To take a screen-shot. Using a Mac, press control-command-shift-4 and use your mouse to select what to copy to the clipboard. Paste into your document.
Using a PC, press alt-PrtScn the active window will be copied to the clipboard. Paste into your document.]
Final Paper Important Informa? on:
- Required Programs:
- ChemDraw
- SciFinder
Acceptable Journals for Reaction Precedent:
- Journal of the American Chemical Society:
http://pubs.acs.org/journal/jacsatLinks to an external site. - Journal of Organic Chemistry; http://pubs.acs.org/journal/joceahLinks to an external site.
- Organic Letters; http://pubs.acs.org/journal/orlef7Links to an external site.
- Journal of Medicinal Chemistry;
http://pubs.acs.org/journal/jmcmarLinks to an external site. - Angewandte Chemie International Edition;http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1521-3773Links to an external site.
- Chemical Science; http://pubs.rsc.org/en/journals/journalissues/sc#!recentarticles&allLinks to an external site.
- Organometallics; http://pubs.acs.org/journal/orgnd7Links to an external site.
- Nature; http://www.nature.com/nature/current_issue.htmlLinks to an external site.
- Science; http://www.sciencemag.org/content/currentLinks to an external site.
- Journal of Natural Products; http://pubs.acs.org/journal/jnprdfLinks to an external site.
- Tetrahedron; http://www.sciencedirect.com/science/journal/00404020Links to an external site.
- Tetrahedron Asymmetry; http://www.sciencedirect.com/science/journal/09574166Links to an external site.
- Tetrahedron Letters; http://www.sciencedirect.com/science/journal/00404039Links to an external site.
- Synthesis; https://www.thiemeconnect.com/products/ejournals/journal/10.1055/s- 00000084Links to an external site.
AN EXAMPLE PAPER IS ATTACHED BELOW
Madesh Veeranna
Total Synthesis of Arboridinine
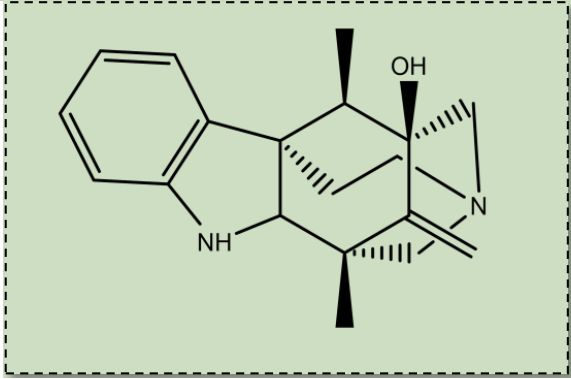
Background/Introduction:
Arboridinine is a pentacylic monoterpenoid indole alkaloid that is derived from a pericine precursor. Arboridinine contains an unprecedented pentacyclic cage skeleton, but shows no biological activity. Arboridinine is the only known monoterpenoid indole that contains pentacyclic skeleton. Arboridinine is unique due to it containing two azepane, as well as a cyclohexyl, and a piperidine ring.
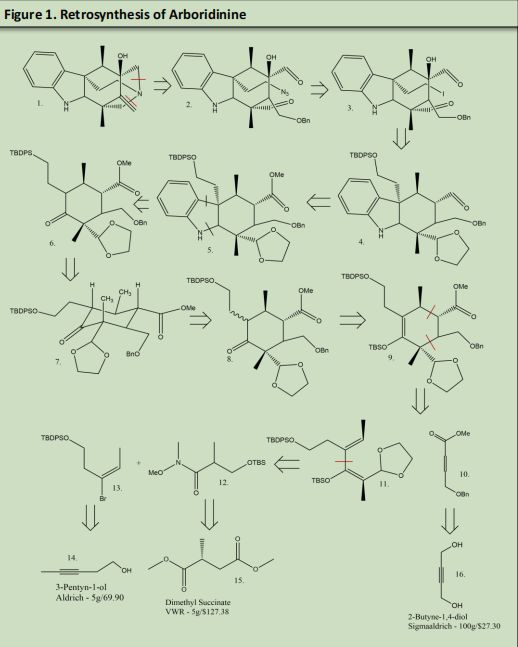
Retrosynthesis:
The bonds that were initially chosen were the two bonds both connect to the amine located at the center of the two seven membered azepane rings positioned in the back of arboridinine. Precedent was found for a double cyclization between two aldehydes and an azide. Azide formation could be achieved by reacting NaN3 with any halogen. This synthesis forms both azepane rings in one step rather than a multistep reaction.
The next bond was that was targeted was the bond between the 6 membered ring and the indole. The three rings were formed using fisher indole formation leaving a ketone bound to the 6 membered ring. Using a Diels-Alder reaction the 6 membered ring could be broken down into a four plus two reaction. The issue that presented itself was the formation of two isomers due to the fact that it is difficult to have high selectivity when protonating an alkene.
The 2 membered alkyl chains were easily formed using 2- methoxyfuran as a starting material. The four membered alkyl chain was assembled using a Grignard reaction between products 12 and 13. The formation of Product 12 and 13 was done using 2- pentyn-1-ol and Dimethyl Succinate. All three starting materials are commercially available. Though the route has many steps the majority of the product can be formed in three separate containers and then combined forming the six membered ring and setting stereochemistry in the process of ring formation.
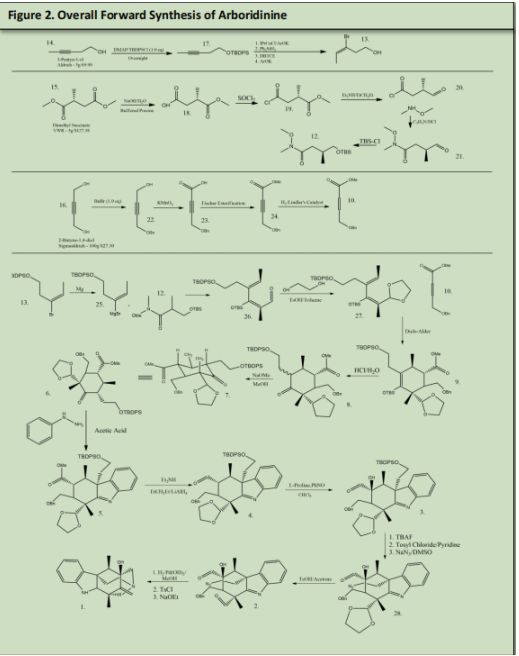
Forward Synthesis:
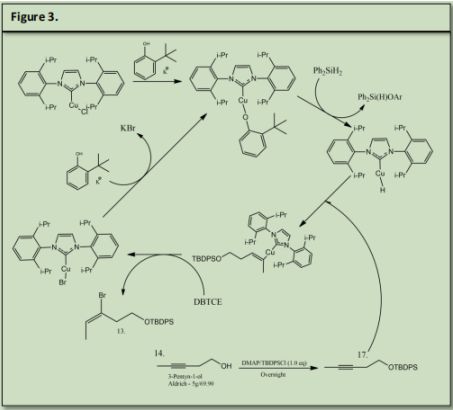
The synthesis of Arboridinine starts with 3-Pentyn-1-ol and is reacted with DMAP and tert-Butyl chloro)diphenylsilane to protect the primary alcohol. Simultaneously Chloro[1,3- bis(2,6-diisopropylphenyl) imidazole-2-ylidene]copper (I) was reacted with Potassium Phenoxide and Diphenylsilane to form a coper hydride. 17 was introduced into the newly formed Copper Hydride to produce an alkenyl copper intermediate. 1,2-Dibromotetrachloroethane was used to perform an electrophilic bromination of the alkenyl copper intermediate and copper bromide.
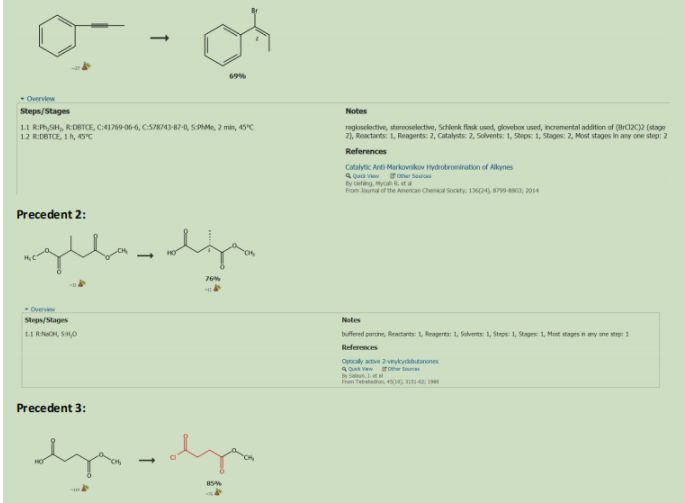
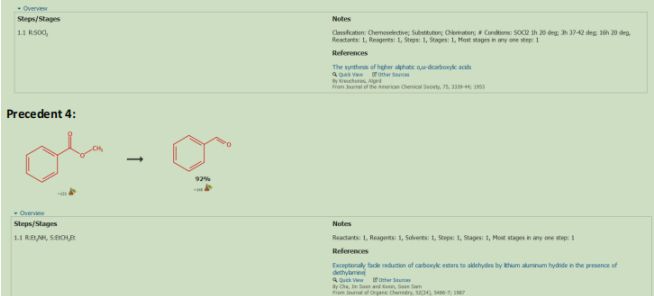
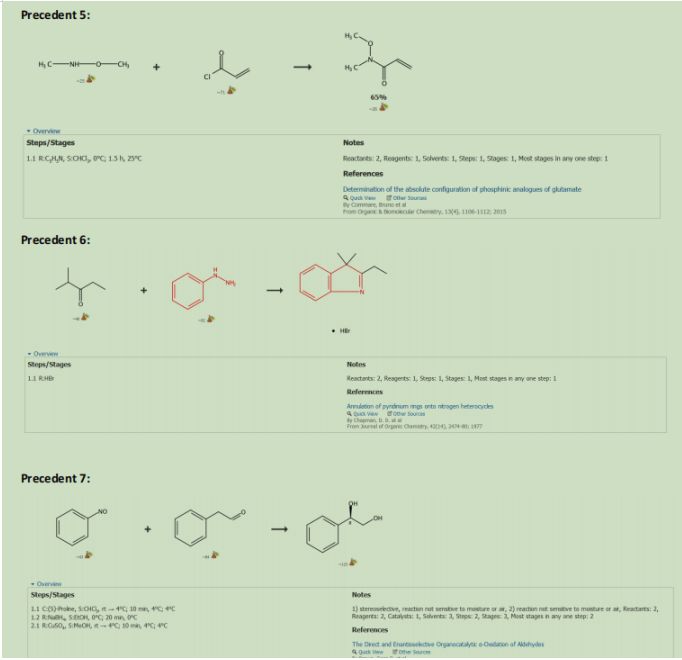
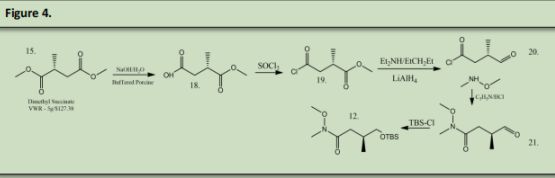
The Catalyst was recovered by reacting the coper bromide with phenoxide via ligand substitution (See Figure 3.) (See Precedent 1)Dimethyl Succinate (15) was reacted with Sodium Hydroxide and water to form 18 (precedent 2). 18 was reacted with Thionyl Chloride to form 19 (precedent 3). 19. Was reacted with Diethyl Amine and Lithium Aluminum Hydride to make 20 (precedent 4). 20 was reacted with methoxymethyl amine to form a Weinreb Amide that will be used as a leaving group later in the synthesis (21) (precedent 5).
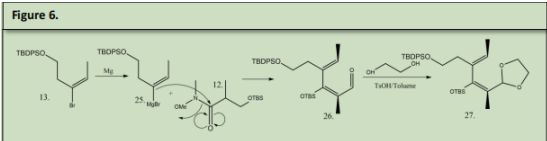
The aldehyde was protected with tert-Butylmethylsily2-Butyne-1,4-diol (16) was reacted with benzyl bromide in mild conditions to protect one of the 2 alcohols (22). 22 was reacted with KMnO4 to form a Carboxylic Acid to form 23. 23 underwent a Fischer esterification to make the carboxylic acid into and ester (24). 24 was underwent hydrogenation conditions using Lindlars palladium catalyst to reduce the alkyne into an alkene. This alkene will be used for the Diels-Alder reaction in a later step. 13 was reacted with magnesium to form a Grignard which was then reacted with the Weinreb amide in 12 to form 26 and sets the stereochemistry of the two methyl groups Alpha and Beta to the OTBS group. 26 was reacted with Tosylic Acid, 1,2-ethanediol and toluene to form a cyclic acetal beta to OTBS to form 27. 27 will be reacted with 10 in a Diels-Alder reaction.
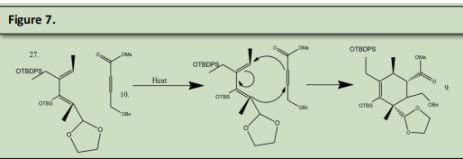
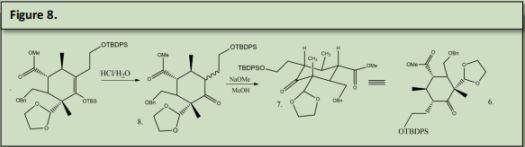
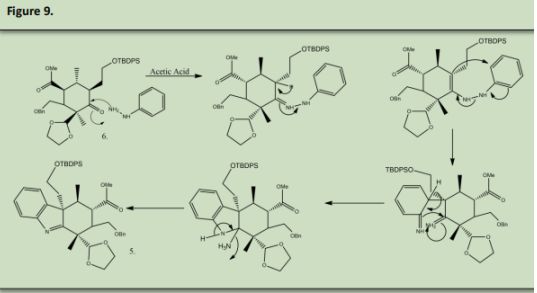
Product 27 and Product number 10 undergo a DielsAlder reaction to form product number 9. 9 was reacted with HCl in water to remove the OTBS group and protonate the double bond simultaneously. This protonation formed two isomers with the chain that is alpha to the deprotected ketone formed in 8. 8 was reacted sodium methoxide in methanol to form 7. Both 6 and 7 are the same produc6 was reacted with Phenyl hydrazine in acetic acid to undergo a Fischer-indole reaction with the ketone that was made in figure 8 product 8 to produce 5. (See Precedent 6).
The Phenyl Hydrazine under a condensation reaction and then is rearranged
The intermediate rearranges once again to form a new carbon-carbon bond, known as a sigmatropic shift. The final rearrangement occurs to restore the benzene ring and closes the five membered ring with the loss of ammonia.
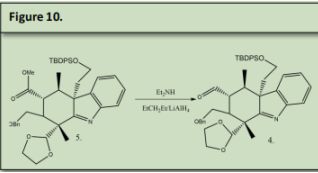
5 (figure 10) was reacted with Diethyl Amine and Lithium Aluminum Hydride (see precedent 4) to reduce the ester that is tertiary to the benzyl ether to an aldehyde. This aldehyde will be later reacted that to form one of the two azepane rings in the final product of arboridinine.
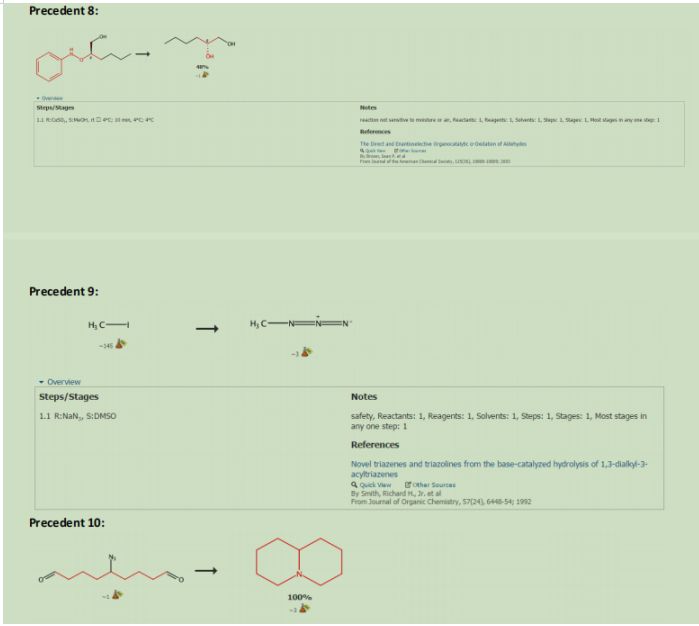
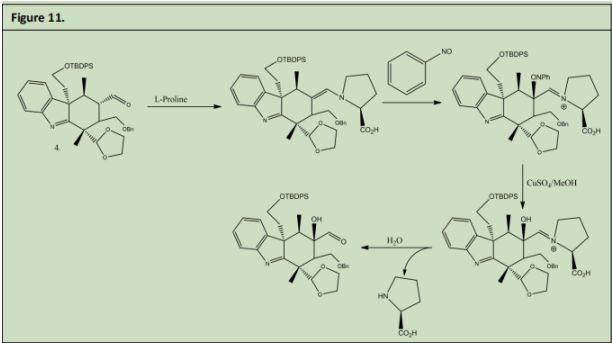
4 (Figure 11) was reacted with L-Proline to form an E-enamine. This enamine was reacted with nitosobezene to form carbon oxygen bond alpha to the enamine. Due to the catalyst chosen the Nitrosobenzene approaches from the front plane making the new carbon oxygen bond out of the plan. This product was then reacted with Copper sulfate in methanol to reduce the Nitrosobenzene to an Alcohol. The subsequent product was reacted with water to reduce the enamine back to an aldehyde and recover the L-Proline catalyst in the process
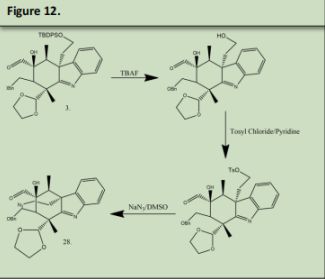
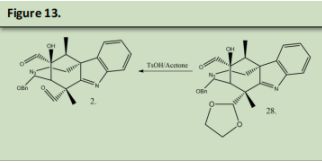
3 was reacted with TBAF to deprotect Alcohol that was protected in figure 3 product 13, and then Tosyl Chloride in pyridine to reduce the alcohol into a Tosylate which will be used as a leaving group in the subsequent step. The Tosylate was remove by reacting the product with Sodium Azide in DMSO (See precedent 9). This azide will be used to form the nitrogen in the double azepane ring. 28 was reacted with Tosylic Acid and Acetone. Tosylic acid was used to deprotect the second aldehyde that was protected in figure 6 product 27. This aldehyde will form the second azepane ring in the arboridinine structure.
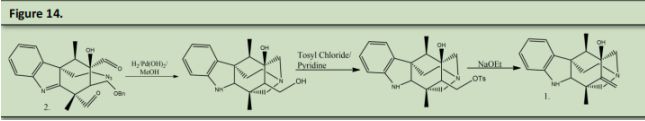
Finally hydrogenation conditions were used with 2 to not only reduce the benzyl ether to an alcohol and to form the double azepane rings. The primary alcohol was reacted with Tosyl Chloride and pyridine followed by Sodium Ethoxide to undergo an E2 elimination to form the alkene.
Supplemental Information:
Precendent 1: