Biomethane from RO Brine via Methanosarcinaceae
- Subject Code :
BPRM430
TRWAssignment
Topic:BiomethaneProductionfromBrineConcentratefromReverseOsmosisusing organisms from the family Methanosarcinaceae
Group No.4
Section1
Instructor: Dr SayantanChakraborty
GroupMembersIDNumber
- MAHIL ANANDHI R 2023AAPS0907U
- LIANA PEARL D SOUZA 2024A1PS0494U
- SHRUTIKA NILESH VICHARE 2024A2PS0014U
- MANUSHREE SIVARAMAKRISHNAN 2024A9PS0365U
- RAKSHA MANIGANDAN 2024A9PS0369U
- DIANAKAVALAGEORGE 2024A9PS0387U
- SAAI DHARSHINI RAJESH VINODHINI 2024A9PS0554U
BioNova Industries
SharjahUnitedArabEmirates
AReport on
BiomethaneProductionfromBrineConcentratefromReverseOsmosisusing organisms from the family Methanosarcinaceae
Preparedfor
Dr.SuzanneHills Director General
By
Dr. Gregory Wilson Head,ResearchDepartment
Approvedby
Dr.SuzanneHills Director General
November 2024
ABSTRACT
Reverse Osmosis (RO) Desalination is one of the primary methods to produce desalinated water, especially in countries where there isaseverescarcityoffreshwater reservoirs. UAE is one of the leading giants in desalination technology, with over 140 operational desalination plants that contribute to 42% of the total water requirement of the country and 14% of the worlds total desalinated water. However, there is a rising concern regarding the largeamountofbrinerejection(alsoknownasbrineconcentrate) that is produced by these massive plants. Like many other countries, UAE primarily disposes its brine into the surrounding water bodies. This disposal of brine causes severe consequences to the marine ecosystem due to the difference in pH and salinity of the brine when compared to normal ocean water. Studies have also suggested that prolonged disposal can negativelyimpacthumanlifeaswell,duetotheaccumulationof toxins through biomagnification. Due to these concerns, the importance of arenewable and sustainable method of recycling the brine produced has come into picture. This study explores the use of four species from the family Methanosarcinacae to produce biomethane from the brine concentrate. Methane in its purified form is a renewable source of energy that has high calorific value (around 50,000 kJ/kg or 11950 kcal/kg) which can act as a fuel substituting diesel and natural gas, which aregenerallyusedto power the RO plant. The presence of CO2and other trace amounts of organic compounds such as DMA (Dimethylamine), TMA (Trimethylamine) and Methanol in brinerejectcanfacilitatemethaneproductioninthesemicroorganisms.Thesalinitylevel of the brine reject produced by all the major RO plants in the UAE was explored and matched with the optimumgrowthconditionsofthefourspecies.Thisnovelmethodcan preventenvironmentaldamagecausedbytheexistingmethodofbrinedisposalandcan also reduce the carbon footprint of the desalination plants across the country.
Keywords - Brine management, biomethane, carbon footprint, methanosarcinaceae, reverse osmosis
ACKNOWLEDGEMENTS
Firstly, we would like to express my heartfelt gratitude to Prof. Souri Banerjee, Director of BITS, Pilani Dubai Campus, who has given us an opportunity to apply andunderstand our engineering concepts in a practical atmosphere.
We would alsoliketothankourfacultyincharge,Dr.SayantanChakrabortyforhisideas and concepts which helped us to analyse research articles and approach the research gap identified in this project, and also to Dr. Shazi Shah Jabeen for assisting us in understanding the structure of a report and presentation.
We are also indebted towards Dr. Neeru Sood,Dr.MurchanaChangmaiandDr.Souvik Kumar Paul for their guidance towards understanding of the microbial processes, the chemical compositions of the brine concentrate and the recommendations forresolving the detrimental effects of brine disposal, respectively.
Last but not the least, we would like to express our appreciation towards our family, friends and team members for their enduring support, encouragement and constant reassurance.
TABLEOFCONTENTS
Abstracti
List ofFiguresiv / iii
- Introduction 1
- Discussion 6
- Seawater Desalination
- TechniquesforSeawaterDesalination
- Seawater Desalination in the UAE
- SWRO plants in the UAE
- Management of Brine concentrate in the UAE
- HarmfulEffectsofBrineConcentrateDisposal
- Impact on Marine Life
- Brine Sinking patterns in The Arabian Gulf
- Marine Organism Tolerances
- ImpactonVariousMarineSpecies
- Phytoplankton
- Benthicforaminifera
- Posidoniaoceanica
- EffectsonDiverseMarineFauna
- EffectsonHumanLife
- Production of Biomethane from RO concentrate
- Adaptations and Characterisations of Genus Methanohalophilus
- Methanohalophilus mahiinov
- Methanohalophilus zhilinae sp.Nov
2.4.1.3 Case study: Lake Elton saline aquatic system
- Adaptations and Characterisations of Genus Methanococcoides
2.4.2.1Methanococcoidesmethylutens
- Metabolic pathways and energetics of methanogenesis
2.4.3.1Methylotrophic pathway
2.4.3.2Hydrogenotrophic pathway
- Storage and Use of Biomethane
- Methods of Storage
- Using Biomethane to Power the RO Plant
2.6 .Results
- Conclusions
- DifferentpracticesofDesalination
- Seawater Desalination in the UAE : Reverse Osmosis
- Impacts of Brine Concentrate Disposal
- Production of Biomethane from RO Concentrate
- Storage and Use of Biomethane
- Results and Conclusions
- RecommendationsReferences BibliographyGlossary
LIST OFFIGURES
Figure 2.1 Desalinationtechnologies analysed using data-driven methods
Figure 2.2 Diagrammatic representation of RO desalination
Figure 2.3 Diagrammatic representation of seawater reverse osmosis
Figure 2.4 Location of major desalination plants in the UAE
Figure 2.5 SWRO in Fujairah plant
Figure 2.6 Salinity indices of 11 RO plants in the UAE
Figure 2.7 Changeininternalosmoticconditionswithexternalvariablesin osmoconformers and osmoregulators
Figure 2.8 Graphillustratingphytoplanktonresponsetoincreasing salinity and pH
Figure 2.9 Representationofincreasedsalinitysimpactonbenthic foraminiferaabundance
Figure 2.10 Representationofthesamplingcampaignscarriedoutattheoutfall areas of Ashkelon, Hadera and Sorek desalination plants to study the impact of brine onbenthic foraminifera
Figure2.11 Potentialimpactofbrinessalinityon flora
Figure 2.12 Picturization of seagrassPosidonia oceanicaseedlings after
7weeksexposuretobrines(0,25,50and100%)showing reduced leaf shoot and root growth.
Figure 2.13 Impact of brine onPosidoniaoceanica
Figure 2.14 Representationofastudyshowingtheeffectofincreasing salinity on the Japanese medaka
Figure 2.15 Potentialimpactsofbrinessalinityonfauna (vertebrate and invertebrate)
Figure 2.16 Flowchart illustrating the steps of the proposed model
Figure 2.17 Variouspathwaysinvolvedinmethaneproductionfrom methylated compounds
Figure 2.18 Steps involved in hydrogenotrophic methanogenesis
Figure 2.19 Schematicofstoragesystemforcompressed Biomethane
Figure 2.20 Schematic to show storage of liquid Biomethane
Figure 2.21 Rectilinear graph comparing the salinity of brine concentrate from11ROplantsintheUAEwithoptimumsalinitiesofvarious Halophilic methanogens
LIST OFTABLES
Table I Summary of various seawater desalination techniques
TableII Advantages & Disadvantages of Evaporation Ponds
TableIII Possible Extended Benefits of Evaporation Ponds
Table IV Conventional brine disposal techniques
Table V Composition of SWRO brine concentrate
Table VI Characteristicsofdifferentstrainsandspeciesingenus Methanohalophilus
Table VII Descriptiveandcatabolicfeaturesofspeciesingenus Methanococcoides
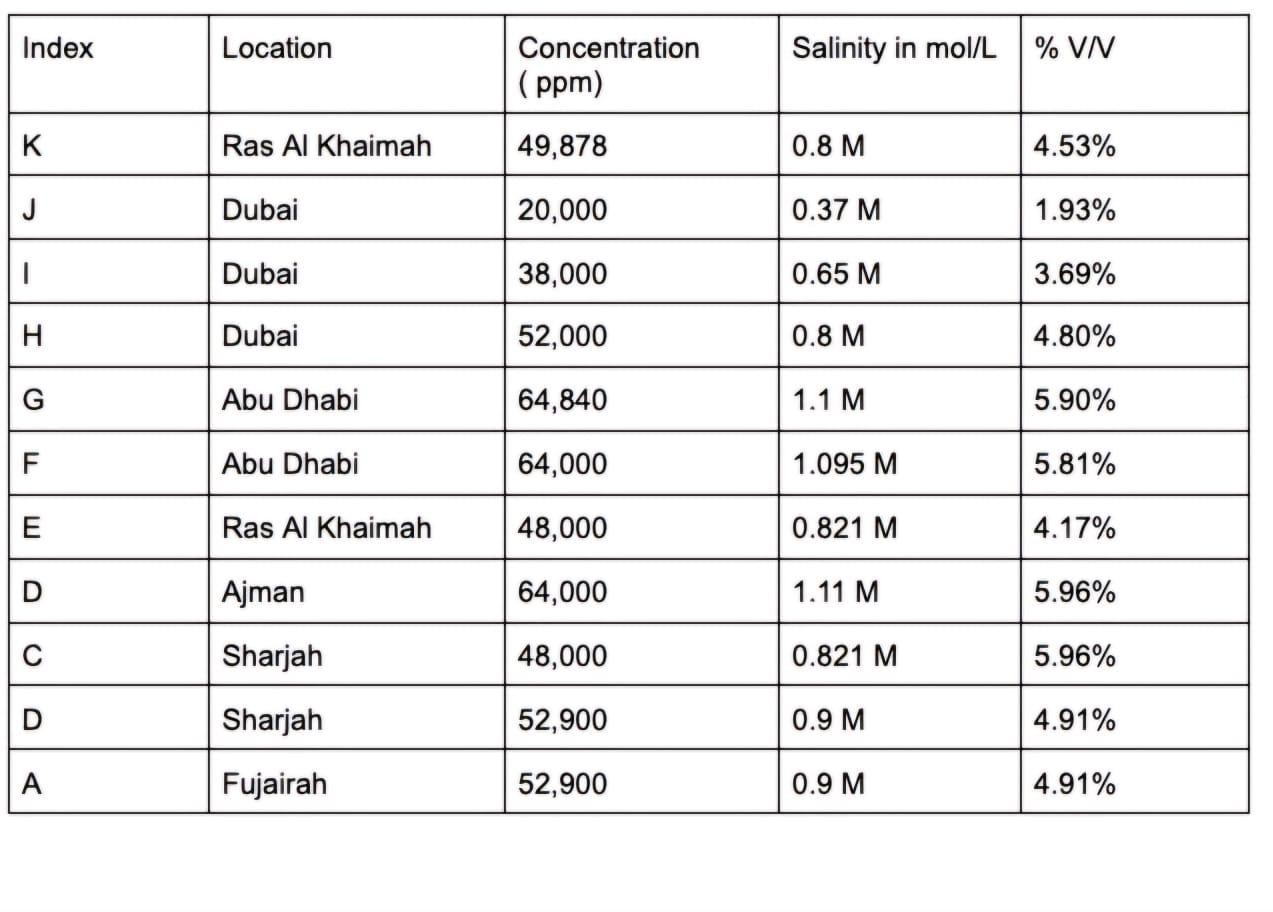
TableVIII Salinityof11ROplantsacrosstheUAEinppm,mol/Land%v/v
CHAPTER 1:INTRODUCTION
- ?AUTHORISATION
This report Biomethane Production from Brine Concentrate from Reverse Osmosis using organisms from the family Methanosarcinaceaehas been authorised by Dr. Suzanne Hills, the Director General of BioNova Industries, on 10th August 2024, to present an innovative strategy to improve existing brinewater treatment technologies by using biomethane as a renewable source of energy.
1.2.?BACKGROUND OF THESTUDY
As the United Arab Emirates (UAE) has limited natural water resources, the water consumed is mainly desalinated, which involves removing salts from water to produce water that meets the quality (salinity) requirements of different human uses.Desalinationiseitherdependentonelectricityin case of reverse osmosis (RO), or a by-product of electricity generation through multiple-effect distillation (MED) and multiple-stage flash distillation (MSF) [1]. Seawater desalination can extend water supplies beyond what is available from the hydrological cycle, providing an immense, climate-independent and steady supply of high-quality water [2]. However, most of the reverse osmosis plants dispose of their brine into the sea, or in nearby creeks that are linked to the sea. There hasnt been enough studies conducted to propose proper solutions for brine concentrate management, and any existing chemical treatment methods arent very sustainable and still cause harm to the environment.
Research [3] shows that as brine is highly alkaline due tocalcium carbonatesand sulphates, and contains chemicals likechlorine,coagulants,antiscalants,etc,whicharetoxic pollutants, it is harmful to marine creatures, degrades water quality and local hydrography, interferes with physiological processes and increases susceptibility to diseases, thereby affecting human life as well. Plankton, bacteria, and benthic species are among the marine animals impacted by rising salinities. The osmotic balance is disturbed, affecting certain marine creatures' regulatory capacities, which can lead to dehydration and, ultimately, mortality.
To resolve this problem, the brine produced can be utilised for a more significant and sustainable purpose which will also help the overall process of reverse osmosis. This involves the production of biomethanefromhalophilicmethanogensculturedinbrine,to provide energy to drive the RO plant.
This study aims to represent abetterstrategytomanagebrinefromdesalinationplants, with a particular focus on using it to produce energy. By doing so, we hope to provide interventions and policies directed at promoting a sustainable environment.
1.3.?OBJECTIVES
The projects aims to:
- ToidentifyissuesregardingtheprocessofReverseOsmosis
- Toanalysethesignificanceofbrine
- Tounderstandtheimpactofbrinedisposalinmarineecosystemsand public health.
- To recommend solutions to enhance water purity by reducing the volume of waste brine.
- Toresearchandoffer methods to improve brine treatment technologies, hence enabling a sustainable and cost effective management.
1.4.?SCOPE
The scope of this study comprises the following:
- It studies the composition andthesalinitylevelofbrinebytakingthedesalination plants present across the UAE into account.
- It focuses on the production of biomethane from methanogens .
- The study considers ten organisms from the family Methanosarcinaceae, while primarily focusing on four of them for the production of biomethane that is to be used for powering up the RO system.
- It covers the process of methanogenesis, for the degradation of compatible solutes for the production of biomethane.
1.5.?LIMITATIONS
The limitations of this study are:
- It focused only on the composition and salinity of the brine concentrate with reference to desalination plants in the UAE and couldnt account for variations within plants in other countries.
- Methanogens do not directly contribute to energy-efficient salt removal and the duration of their survival in highly saline water is unreliable and hasnt been physically experimented within a lab setting.
- The costs involved in the extraction of these biological organisms has not been calculated due to lack of time and research on the topic.
- The methane gas that the microorganisms produce couldcauseclimatechange, which indicates that the amount of methanegasproducedneedstoberegulated so as to not be overproduced.
- Methane gas is highly flammable and thusneedsproperinfrastructuretocontain
- This is a theoretical model and doesnt yet have any lab results to confirm its efficiency and has been proposed based on the general behaviour of methanogens. Some methanogens might find it difficult to survive in the toxic conditions of the brine concentrate.
- Methanogenesis is often a slowprocessthatdependsoncomplexenvironmental factors (such as temperature, pH, organic material availability, and oxygen levels), which requires digital simulations and in-field experiments to determineits complexity and cost.
1.6.?METHODSANDSOURCESOFDATACOLLECTION
This report is based on data collected using a combination of both primary and secondary data.
Primary data used includes calculations which were required to properly determine the feasibility and practicality of the proposed solution. Secondary data primarily involves the literature review.
The information regarding the composition of brine concentrate and its negativeimpact upon disposal were obtained from genuine publications such IEEE, ResearchGate and government websites such as DEWA and scad.gov.ae.
Information regarding genomic data, metabolic pathways, characterization and extraction of species from the family Methanosarcinaceae were obtained from publications such as ScienceDirect, NCBI PubMed and Frontiers. This guarantees that the information used is unbiased, legitimate and relevant to the time period of our study.
1.7.?REPORTPREVIEW
This report consists of 4 chapters:
Chapter 1-This chapter gives us anintroductiontothetopicofresearchanddiscusses how desalination, although a primary method of treating saline water to produce freshwater, has negative consequences due to the unsafe disposal of its main waste product, brine. The background of the study also tells us how this problem could plausibly betreatedandthrowslightontheobjectives,scopeoftheresearch,limitations and sources of the data provided.
Chapter 2-This chapter takes us a step forward and tells us about how brine is currently disposed of and its detrimental effects on the ecosystem delving into the research gap identified in section 1.2 of the first chapter.
Chapter 3-Chapter 3 has conclusions drawn from the above discussions about seawater desalination,currenttechniquesandbrinedisposalmethods.Thischapterhas inferences on how biomethane could be produced using methanogens and effectively be put to use.
Chapter 4-This chapter includes recommendations which could effectively solve problems mentioned in Chapter 2 of discussion, including, the effective conversion of brine into an environment-friendly and sustainable resource, thereby aligning these solutions to the Sustainable DevelopmentGoals(SDG)proposedbytheUnitedNations (UN)
CHAPTER 2:DISCUSSION
This chapter explores the different practicesofseawaterdesalination,thedominanceof Reverse Osmosis in desalination, currentdesalinationtechnologiesintheUAE,andthe currentmethodsofbrinedisposal.Italsoexplainsthedetrimentaleffectsofthisdisposal on both themarineecosystemandhumanlife,expandingontheresearchgapidentified in section 1.2 of Chapter 1. A theoretical model is proposed in this chapter to mitigate the harmful effects ofbrinedisposalintotheocean.Thegenomicandmetabolicdataon the Methanosarcinaceae family,whichincludehalophilicmethanogenicbacteriathatare essential to the process of the model,alongwiththetechniquestostore,distributeand utilise the biomethane produced from the process are also discussed in this chapter.
2.1?SEAWATERDESALINATION
Desalination is the process in which the concentration of salt in saline solution is lowered to a level that can be utilized for human use, the by-product of this process is brine. This process is one of the best solutions to the increasing globalconcernofsafe usable water, which has been highlighted by the United Nations throughSustainable Development Goal 6,aimed at ensuring universal access to clean water andsanitation.
The saline feed-water is taken from the ocean or underground waterbedswhichisthen put into the desalination process wherein it gives two streams of liquid the low-salinity product water and brine (very saline concentration or reject water).
Some substances in seawater, such ascalciumcarbonatecanberemovedbychemical treatment but substances such as sodium chloride need more sophisticated processes such as desalination [4].
2.1.1.?TechniquesforSeawaterDesalination
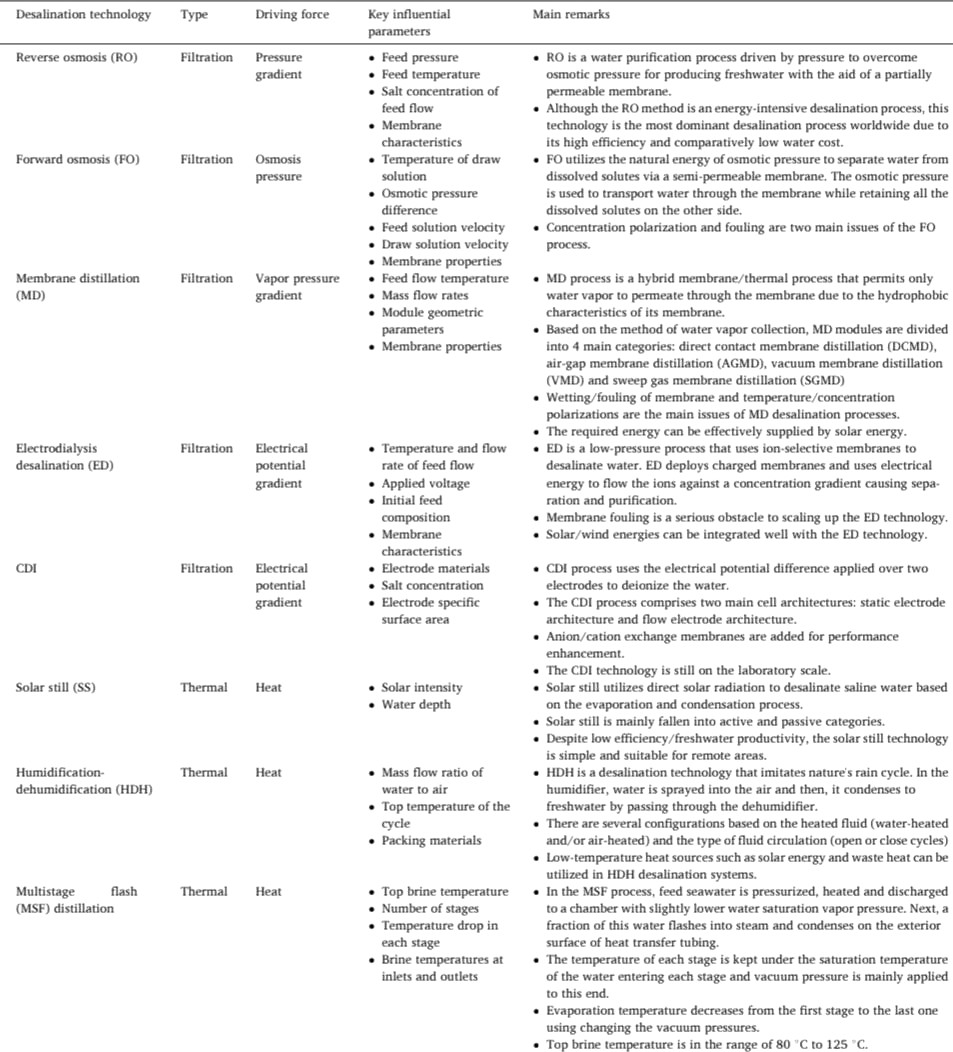
As shown in Fig. 2.1, desalination technologies primarily fall under two categories: thermal and filtration-based processes. Freshwater is mainly produced by vaporization and condensation in the thermal-based processes, as illustrated in Fig. 2.3. In the case of filtration-based processes, semipermeable membranes are employed for freshwater production, except for the capacitive deionization (CDI) desalination method, in which porous electrodes are used for salt removal. Table I also summarises all the techniques [5].
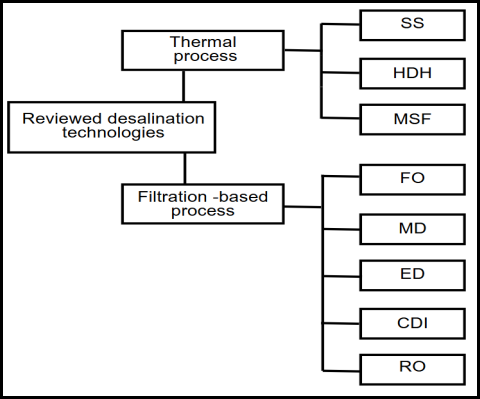
Fig. 2.1Desalination technologies analysed using data-drivenmethods.
?Solar Still(SS):
?Solar distillation uses solar energy to purify water through evaporation and condensation and is carried out by a solar still. Its function is very simple; a transparent cover encloses a pan ofsaline water and later traps solar energy within the enclosure. This heats up the water causing evaporation and condensation on the inner face of the sloping transparent cover. This distilled water is generally potable and the quality of the distillate is very high because all the salts, inorganic and organic components and microbes are left behind in the bath. [6] [7]
?Solar distillation has advantagesofhavingalowoperatingcostand simple device structure, but the drawbacks are that the device space occupancy is large, yield per unit is low, and the thermal property can be influenced by different areas and climate conditions. At present, freshwater produced by it per day is only34 kg/(m2 d). [8]
?Humidification-dehumidification(HDH):
?This method is a thermal desalination technology that imitates natures rain cycle. Its basic components consist of a humidifier, a dehumidifier, and a heater. In the humidifier, air is humidified through direct contact with salty water. In the dehumidifier, hotmoist air is put in indirect contactwithcoldsaltywater.Thiscauses water vapour to condense, which produces a freshwater stream [9].
- Itsadvantagesinclude:thepotentialofutilisingbrine,theabilityof
using low-grade, renewable thermal energies and a high range of salinity from brackishtoseawaterwithevenimprovedperformance at higher salinity, the possibility of providing the requiredenergyby photovoltaic (PV) panels, and simple construction with low-cost operation and maintenance.
?HDH also suffers from some drawbacks such as: low energy efficiency compared to other desalination technologies, low water production scale compared to RO, limited commercial availability, low thermal efficiency due to maintaining the water temperature below the scale formation threshold and, large area requirement leading to high capital cost [10].
?Multistage Flash Distillation(MSF):
- In MSF, a saline solution is heated and flows into a chamber at progressively lower pressures. In the chamber, a portion of the brine flashes into steam. The flashed steam passes through amist eliminator, condenses to pure water, and is recovered as the product water. The product water often requires remineralization because its TDS (210 mg/L) is too low forpotable water. The systems also have a high operating cost when waste heat is not available for the distillation process and relatively high rates of corrosion and scale formation due to high operating temperatures. However many MSF plants have outlived their design lifetime [11].
?MSF systems offer relatively higher energy efficiency and lower energy consumption [12].
?ForwardOsmosis:
?Forwardosmosisusesasemipermeablemembranetocarryoutthe separation of water fromdissolved solutesby having anosmoticpressuregradient.
?Forward osmosis is advantageous as it operates at low or nohydraulic pressures, it has high rejection of contaminants, and it may have a lower tendency ofmembrane fouling. However, there aretwomajorlimitationsofforwardosmosislackof
high-performance membranes and the necessity for an easily separable draw solution. Moreover, when high water recovery is desired in seawater desalination, forward osmosis can be utilised only if the draw solution can generate a high osmotic pressure [13].
?Membrane Distillation(MD):
?Membrane distillation involves a vapour transport driven by the vapour pressure gradient across a membrane that is hydrophobic.
?In this process, theaqueous feed solutionis first heated and brought to thehydrophobicmembrane.Theporesofthemembrane removes the aqueous solution and allows the vapor to pass and condense. The vapor diffuses through the membrane, achieved by avaporpressuredifferenceacrossthehydrophobicmembrane.MD can potentially recover both chemicals and water. However, once the feed solution attains a concentration comprising the maximum solute solubility the membrane can exhibit scaling and wetting, which will reduce themembrane flux[14].
?Electrodialysis Desalination(ED):
?Electrodialysis Desalination uses membranes that only allow the passage of eithernegativelychargedorpositivelychargedionsata time, and it is used to desalinate large volumes of water. Thedriving force behind it is an electrical potential that attracts and moves cations and anions through a permeable membrane, producing fresh water on the other side [15]. ED has high salt removal rates, is less susceptible to scaling and has high segregationof metals [16].
?The maindisadvantagesincludetheneedfordevelopedsystemsof waterpretreatment(upto7stages)andfrequentmembrane
replacement (sometimes after 6 months). The cost of membranesis 50% of the equipment's cost [17] .
?Capacitive Deionization (CDI)Desalination:
?In this method, saline water passes between a pair ofporouselectrodes, which can be static or flow electrodes, and which are usually carbon-based. A differential voltage ofbetween1and1.4V is applied to this pair of porous electrodes; consequently, the salt ions in the feed flow migrate to theelectrical double layers(EDLs) at the carbon/water interface, which performs the desalination.
?There is no need for high-pressure pumps orheatsourcesforCDI. Furthermore, it is suitable for low salinity feeds as salt ions are separated from water, which is similar to electrodialysis. However, one major drawback of this method is that it is not capable of removingimpuritiesotherthanions(suchasviruses,bacteria,etc.). [18]
?ReverseOsmosis:
- Reverse osmosis (RO) is a pressure drivendesalinationprocess wherein a pressure higher than theosmotic pressureof the feed water is applied to it and is allowed to pass through a semipermeable membrane, which enables fresh watertopermeate across the membrane. Today, reverse osmosis (RO)iswidelyused to remove the majority of solutes from water before ion exchange treatment, which significantlyreducesthechemicalsforthefinalion exchange polishing [19].
?RO is the most dominant process used in brackish and seawater desalination and in theproductionofdrinkingwatermainlybecause
it is an energy-efficient technology for desalination, with energy costs and it possess the dual advantage ofmaintaininggoodwater permeability and very high rejection rates for nearly all organic, inorganic and pathogenic micropollutants [20]. Fig. 2.2 shows the workings of RO desalination.
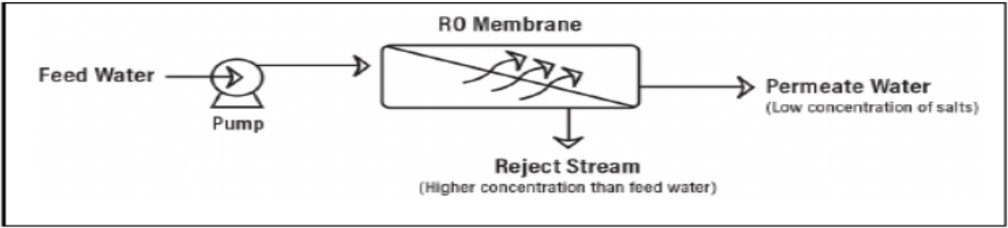
Figure 2.2Diagrammatic representation of ROdesalination.
TableISummaryofvariousseawaterdesalinationtechniques
2.2?DESALINATIONINTHEUAE
Desalinated water and treated-sewage water comprise the non-conventional water resources in the UAE [21], with RO being one of the methods to produce desalinated water as shown in Fig 2.2 and Fig.2.3[22]. At present potable water whichaccountsfor about 42 per cent of the total water requirement, is provided by 70 major desalination plants.
2.2.1?Seawater Reverse Osmosis in theUAE
The concept of seawater reverse osmosis (SWRO) is to use a semi-permeable membrane where most of the dissolved species are rejected while water permeates it [23]. .
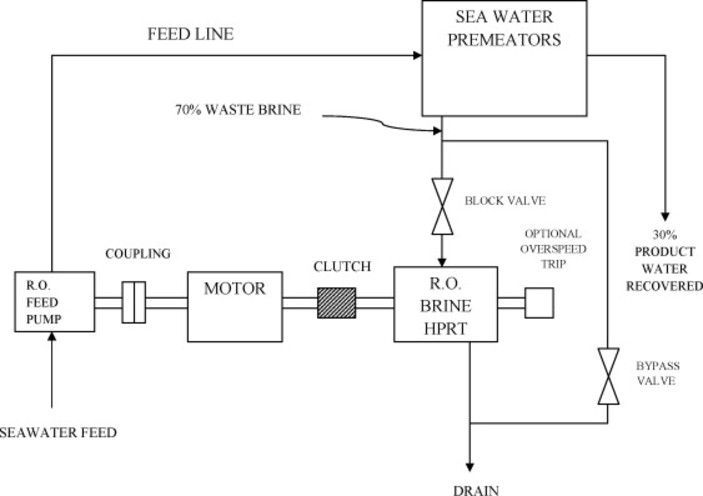
Figure 2.3 Diagrammatic representation of seawaterosmosis.
The UAE obtains 475 Mm3 of desalinated water per year. There are more than 20 Seawater Reverse Osmosis (SWRO) plants across UAE, the 3 major SWRO plants located in UAE (as shown in Fig. 2.3) are:
- Jebel Ali SWRO plant
- Shuweihat SWRO Plant
- Fujairah SWRO
The location of the 10 major desalinationplantsintheUAEareshowninFig.2.3 [24].
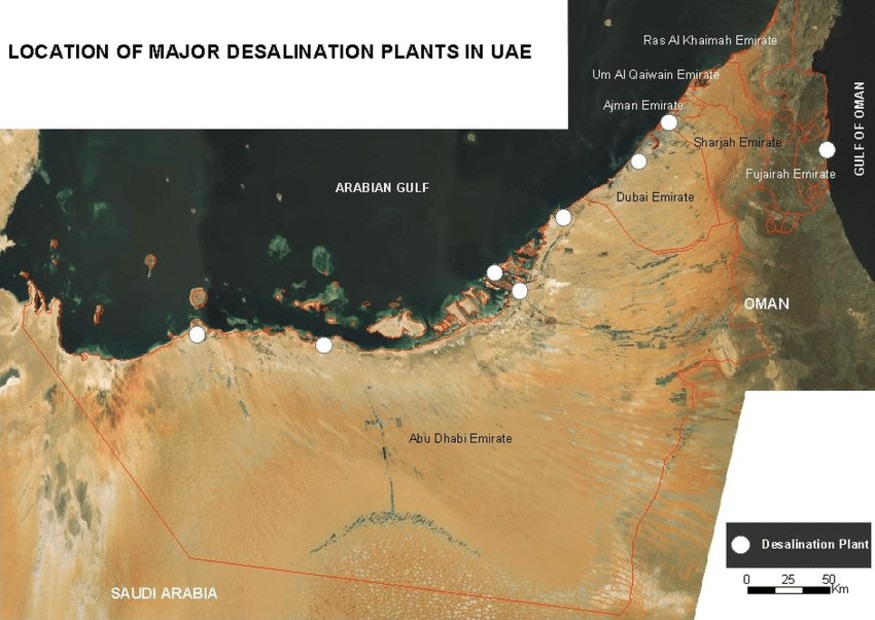
Fig. 2.4Location of major desalination plants inUAE
TheFujairahSWROplantisoneofthelargestdesalinationhybridplantsintheworld. Its working is shown in Fig. 2.4 [25].
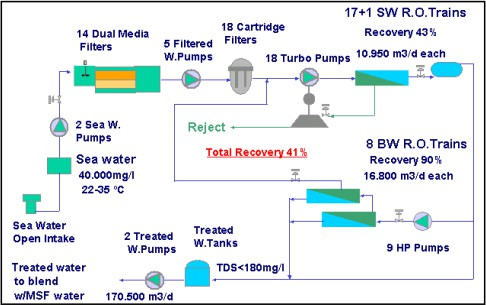
Fig. 2.5SWRO in Fujairahplant
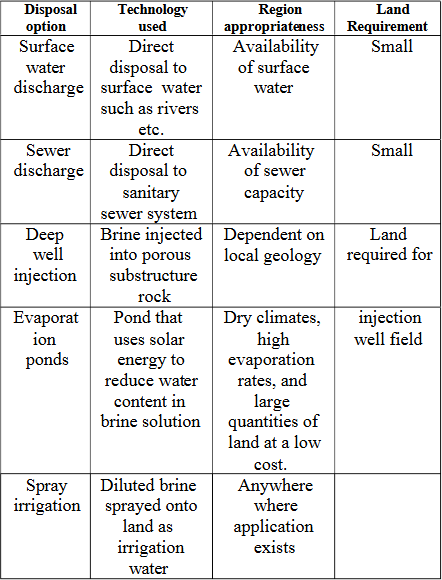
2.2.2?Management of Brine concentrate in theUAE
Brine is often diluted before being disposed back into the ocean (surface water discharge). It doesnt make the brine harmless completely but does lessen its effect on the marine environment.
Inlanddisposal methodsinclude:
- Deep-Well Injection: In this method, the brine is injected deep underground into salt caverns or geological formations, separating it from thesurfaceThismethodisusedinsomecountries,butit'sstill being explored for large-scale application in the UAE.
- Spray irrigation: It is when brine is diluted to be used for irrigation purposesfortheItisnotveryeffectivebutissaidtobeabetter method than disposal of brine into the ocean.
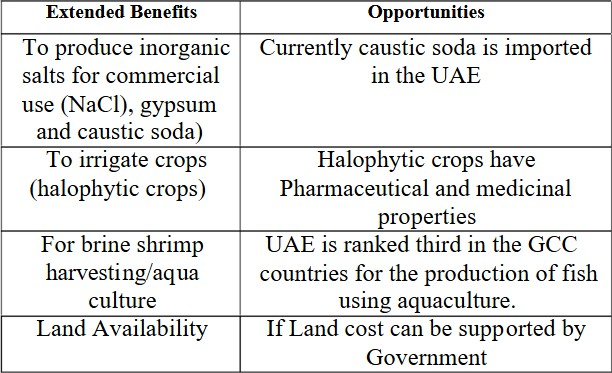
- Evaporation Ponds: It is used to store saline solution and to reduce its volume. It is not currently being used in the UAE due to its cost as compared to disposal to sea [26]. The advantages and disadvantages of evaporationpondsaswellastheextendedbenefitsofevaporationponds are shown in Table II and Table III respectively.
TableIIAdvantages&DisadvantagesofEvaporationPonds
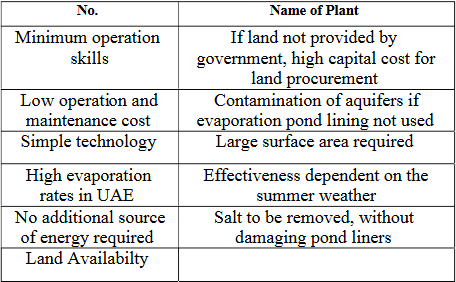
TableIIIPossibleExtendedBenefitsofEvaporationPonds
TableIVConventionalbrinedisposaltechniques
2.3.?HARMFULIMPACTSOFBRINECONCENTRATEDISPOSAL
Utilisingdesalinatedwaterforirrigationcouldcreateadditionalenvironmentalproblems, most notably the potential for gradual degradation of soil structure, this varies from one case to another but desalinated water does contain a higher concentration of sodium and boron ions. Prolonged exposure to excessive amounts of sodium and boron could degrade the physical properties of the soil and cause boron-toxicity damage to the soil [27].
In summer, the seawater temperature is about 35 degree Celsius and the plants can tolerateanincreaseof7to8degreeCelsius.Duetohightemperatureandsalinityinthe water during brine disposal the concentration of oxygen decreases, hence increasing the chlorine concentration which disturbs the ecological system.
Thefollowing arehighly endangeredareas highly affectedby thedisposal of brine:
- Mangrove swamps that are present in the tidal flats and coral
- Seagrass(bothsparseanddense)playanimportantroleintheGulfmarine environment, almost 9% of the Gulfs fauna is dependent on seagrass.
- Marsh (a form of salt that is also called sabkha) which is common in the coastal and inland parts of the UAE. Fig. 2.4 demonstrates the vastly different salinity of brineconcentratecomparedtofeedThesalinityoffeedwaterandofbrine RO plants in the UAE are shown in Fig. 2.6 [28].
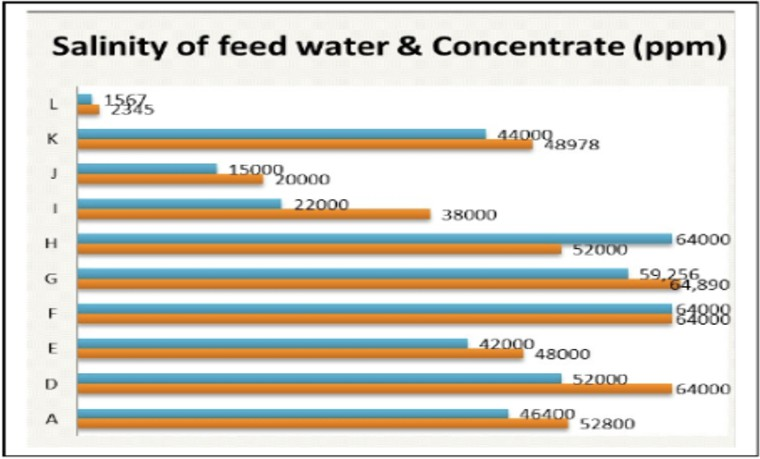
Fig.2.6Salinityindicesof11ROplantsintheUAE
2.3.1.?Impact on MarineLife
In the wake of UAEs aim to expand desalination output by an added 7 billion imperial gallons per year before 2030, we can expect larger volumes of concentrated brine reject tobedisposedof.TheSDGs2030proposedbytheUN has pushed the UAE to explore mitigation techniques to address this rapidly growing problem.
SDG 14 addresses the rapidly declining nature of the marine ecosystem due to human exploitation.Untreated brine waste from desalination plants is the primary source of contamination and destruction of marine flora and fauna. Thesustainable use of brine waste can remarkably decrease the negative impact
on the biosphere, and can allow for the fruitful regeneration of the marine ecosystem to its former potential. The instability of sea-levels can cause many issues in the near future such as unpredictable natural disasters, loss of lifeand property, damage to the economy and reduced accessibility of marine transportation and oversea trade.
The current efficiency of SWRO is 4050%; and the brine waste that isgenerated and discharged into the sea has approximately twice the salinity of seawater. Whats important to note is that the discharge of concentrated brine and chemicals (e.g., phosphonates and ferric sulfate-based coagulants) that are added in the desalination process are very harmful and have detrimental effects on the environment. Desalination brine also contains many chemical hazardous wastes, like antifouling agents, chlorine and acid which are unavoidably needed for the upkeep of pipelines. What is critical is that these constituents are not treated to remove toxicity before being dumped into the sea.
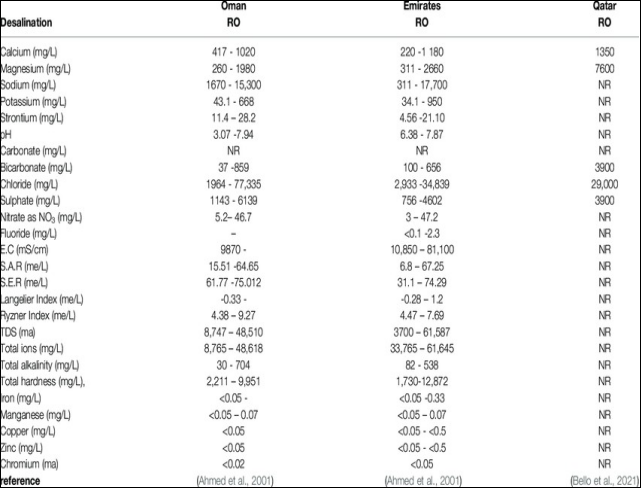
Brine from seawater desalination can also contain high concentrations of elements including heavy metals suchaslead,manganese,copperandzinc[29]. Table V details the composition of brine from plants in Oman, UAE and Qatar.
TableVCompositionofSWRObrineconcentrate
Heavy metals and toxic compounds can be harmful to marine creatures- they degrade the water quality and local hydrography of the receiving water body, interfering with physiological processes ofthebiotopesuchasenzymaticactivity, nutrition, photosynthesis, respiration, and reproduction, due to changes in gas solubility and the occurrence of toxic ions.
Marine organisms are always in osmotic balance with their environment- the osmotic stresses acting on diverse species depend upon individual adaptations and salinity tolerances which vary across specific habitats. It is imperative we study a few of the marine species adversely affected by brine concentrate to devise a solution.
Cost playsapivotalroleinselectingdisposalmethods,anddisposingbrinewater into the sea isaverycosteffectiveapproach.However,dischargingbrinedirectly into the sea can negatively impact several benthic marine organisms thattypically live in environments having stable salinity, with effects ranging from reduced larval survival to complete extinction of the species. The magnitude of this impact depends on the desalination plant and biological conditions of the receiving marine environment. In thissection,wewillexplorethediverseimpacts of brine water on marine life focused in the Arabian Gulf.
2.3.1.1?Brine Sinking Patterns in The ArabianGulf
The Brine discharged from SWRO desalination plants is always denser than the ambient seawater with respect to higher salt loads, but due to elevated temperatures, it displays a reduced density, almost equal to that of open sea orseawaterintake,andconsequentlyhasnegativebuoyancy. So, the dissipation of effluent depends largely on the bathymetry of the discharge site, prevailing tides, currents, wavesandwindintensitiesinthe outfall zone. The brine discharged from plants, rapidly sinks in the outfall zone, attaining rapid dilution in an area immediately after the discharge point.
The salinity of brine is reduced by diluting it with regular seawater, municipal wastewater, and cooling water from a neighbouring power station prior to disposal[30]. However, because the temperature and salinity of water discharged from the desalination plants is both higherthan that of the receivingseawater,thedischargecanfloatonthesurface. But, as we established before, due to heavier and higher salinity of discharge, it also drags the cooling power plant water with it as it descends.
We must also be cognizant of the marine organism salinity threshold tolerances in order to ascertain the impacts of brine water on marine life.
2.3.1.2?Marine OrganismTolerances
Temperature and salinity are some of the most important environmental variables governing the survival and development of marine species. Organisms usually have a specific and relatively constant internal concentration of salts, which can however be influenced and altered by external changes to the salinity of the aquatic environment. Marine organisms have 2 strategies for coping with salinity changes:
- Osmoconformers: Echinoderms and other invertebrates are includedunderthisThesehaveaverylowcapacityto regulate their internal osmotic concentration, whichresemblestheir inhabited environment. Hence, they are very sensitive to minor salinity changes.
- Osmoregulators: Fishes and other vertebrates are included under this classification. They adjust their internal osmotic concentrations by accumulating organic salts within their tissues or excreting selective ions. They have different tolerances to salinity changes, and osmoregulation usually demands a high energy cost, which is reflected in lower growth rates and higher mortalities of organisms when exposed tosalinitylevelsoutsidetheiroptimaltolerance Summarization of differences between osmoconformers and osmoregulators is shown in Fig. 2.8.
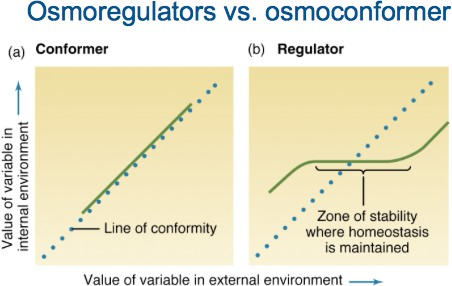
Fig. 2.7Change in internal osmotic conditions with external variables in osmoconformers and osmoregulators.
Some marine organisms can withstand small variations in salinity or occasional exposure to levels higher than the natural environment. However, extreme or abrupt changes can result in physiological stress, causing mortality of smaller organisms.
2.3.1.3?Impact on various marinespecies
2.3.1.3.1?Phytoplankton
Phytoplanktonarecrucialtotheglobalcarboncycle,the foundationoftheoceanfoodweb,controllingthecarbon
dioxide and oxygen levels on Earth. Figures vary, but estimates suggest that they are one ofearthsmajorprimary producers, responsible forup to 85%of the oxygen in the atmosphere and 50% of all photosynthesis on Earth.
It is only reasonable that crashes in their population have seriousconsequencesontheentiremarineecosystem.They can be used as bio indicators to assess the health of an ecosystem. Research was conducted on 3 classes Bacillariophyceae (Diatoms), Pyrrophyta (Dinoflagellates) and Cyanophyceae (Blue green algae) in the Arabian Gulf. An increase in the salt concentration in their environment was noted to reduce their production to extinction (mainly of larvae and young individuals). Some of the species, mainly the diatoms, are resistant to high salinity levels, but most of the species will not survive.
Certain biocides present in the brine also easily kill organisms not targeted, like phytoplankton, the meroplankton, the holo zooplankton and the ichthyoplankton.Fig. 2.7 demonstrates the phytoplanktons response towards the increase in salinity and pH.
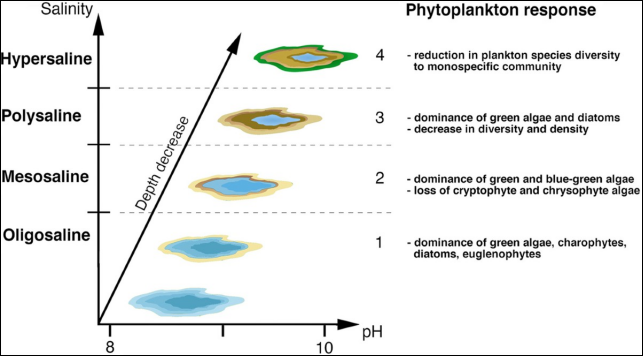
Fig. 2.8Graph illustrating phytoplankton response to increasing salinity andpH.
Heavy metals like Cu or Fe also affect phytoplankton populations. At feeble concentration, phytoplankton utilise these elements in metal-requiring and metal-activated enzyme systems which catalyse major steps in glycolysis, the tricarboxylic acid cycle, photosynthesis and protein metabolism. However, at elevated levels, Cu is very toxic, it acts as an enzyme inhibitor in organisms and can result in the demise of large numbers of susceptible organisms. For phytoplankton, Cu inhibits photosynthesis, restricting the uptake and assimilation of nitrate and the uptake of silicate. There is a noteworthy decrease in the abundance and diversity of phytoplankton species.
2.3.1.3.2?Benthicforaminifera
Benthic foraminifera are a known bio-indicatorverysensitive to physicochemical and biological characteristics like seawater temperature, salinity, pH and dissolved oxygen levels. Therefore, the characteristics of the benthic foraminiferal tests provide valuable information about past climatic and oceanographic changes, such as sea level, monsoon intensity, temperature, salinity and ocean circulation. Shown in Fig. 2.8 is the impact of salinity on benthic foraminifera abundance.
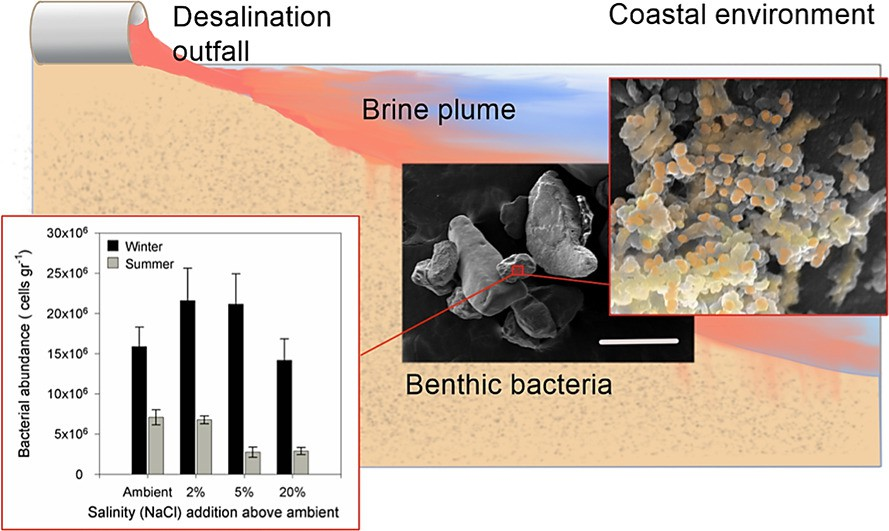
Fig.2.9Representationofincreasedsalinitysimpactonbenthicforaminiferaabundance.
Seasonal changes in benthic foraminiferal assemblages were monitored at three desalination facility sites(Ashkelon, Sorek, and Hadera) along the Mediterranean coast of Israel during 20162017[31]. The most noticeable effects of the brine and temperatures were the decreases in abundance and species richness observed between most of thesamples at the outfall and the control stations.
The Soreksitefacilitatedanisolatedevaluationoftheimpact of elevated salinity on the foraminiferal assemblages. The most noticeable negative response to brine discharge was observed among different lineages of organic-cemented agglutinated species. Fig. 2.9 shows the impact of brine on the outfall desalination plants in various areas.
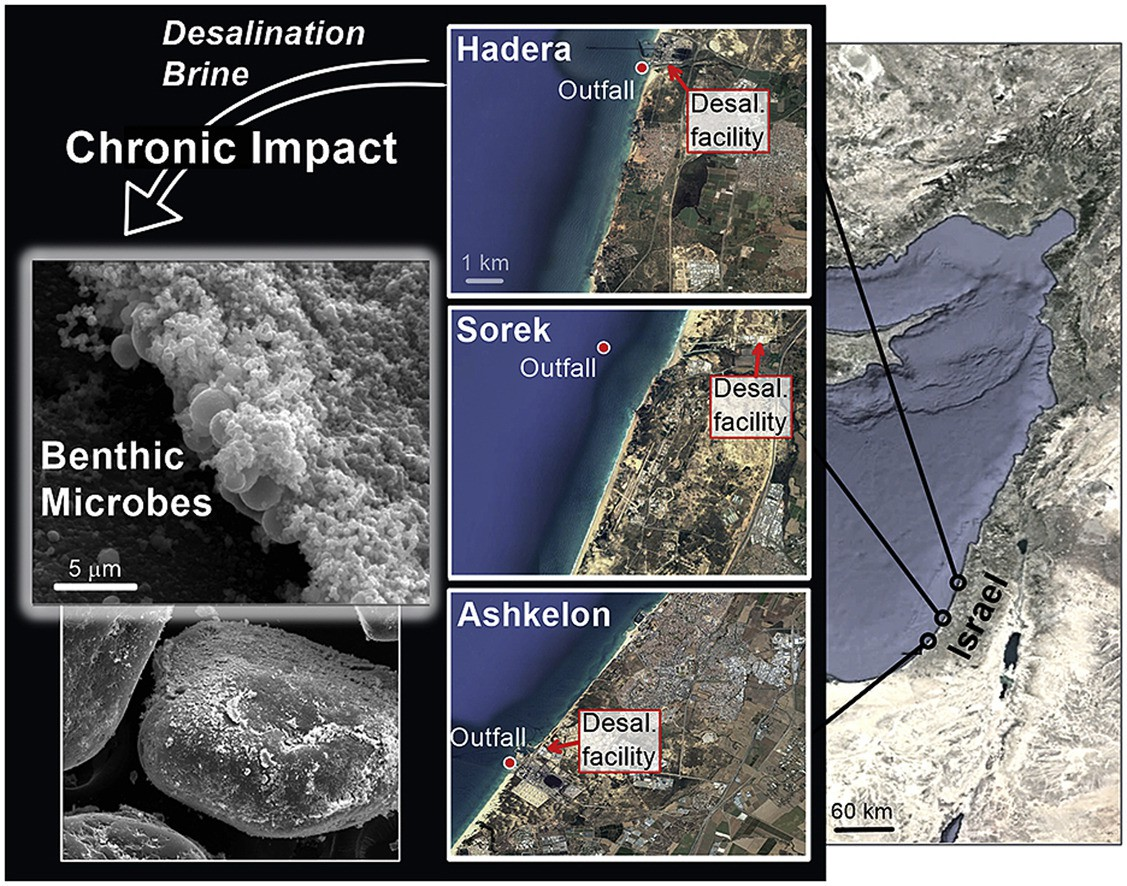
Fig. 2.10 Representation of the sampling campaigns carried out at the outfall areas of Ashkelon, Hadera and Sorek desalination plants to study the impact of brine on benthic foraminifera
This study demonstrates that an increase of 23 salinityunits caused by desalination plants has a minor, local effect on calcareous benthic foraminiferal assemblages and amore prominent impact on organic-cemented agglutinated species[32]. A significant death rate follows increasedsalinity levels.
2.3.1.3.3?Posidoniaoceanica
Desalination brine discharges have severely impactedPosidonia oceanicaor seagrass for a prolonged period [33]. Seagrass plays a crucial role in the marine ecosystem; meadows ofPosidonia oceanicacan shelter algae, invertebrates and vertebrates in areas of high biodiversity, and contribute to improving water quality, preventing coastal erosion, and regulate biochemical fluxes along the coast,and are an integral part of the marine ecosystem. Fig. 2.11 depicts the impact of brine on various florae.
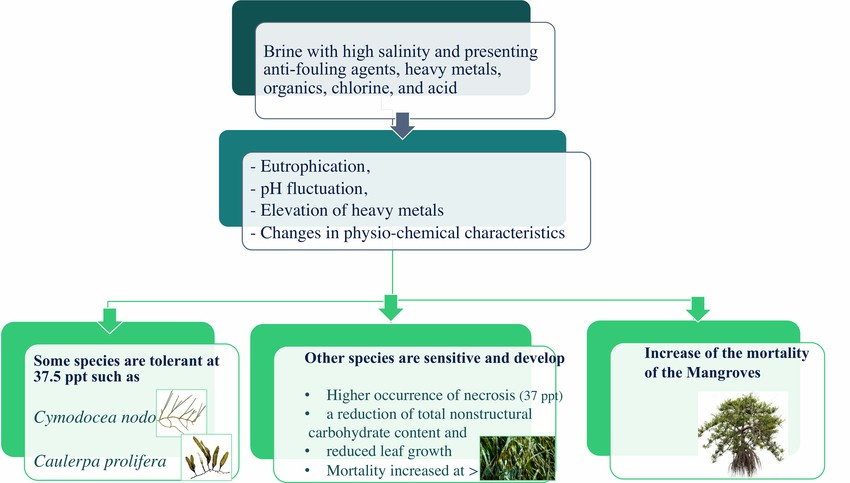
Fig.2.11Potentialimpactofbrinessalinityonflora
Thalassia testudinumwas unaffected by a salinity increaseof up to 4 ppt from an RO desalination discharge, and bothCymodocea nodosaseagrass andCaulerpa proliferagreen algae were unaffected by a discharge plume as long as the salinity remained below 37.5ppt,whichiswithintheirnatural salinity range; whereas a 12 ppt increase above ambient salinity of 3738 ppt caused significant effects onP. oceanica[34].P. oceanicais sensitive to increased salinity due to brine discharge and was adversely impacted, and results showed that the plantstissuessufferedadeleterious increase in nitrogen content and reduced glutamine synthetase activity, harming its health, especially next to the discharge where the salinity is higher. The impacts on the plants health were a higher occurrence of necrosis, a reductionintotalnon-structuralcarbohydratecontent,and
reduced leaf growth. The structureandvitalityofP.oceanicawere severely affected at a salinity of 39.1 ppt and38.4ppt, respectively, and the plantsmortalityconsiderablyincreased above 40ppt,as50%ofP.oceanicadiedupontheexposure to 45 ppt salinity within 15 days.
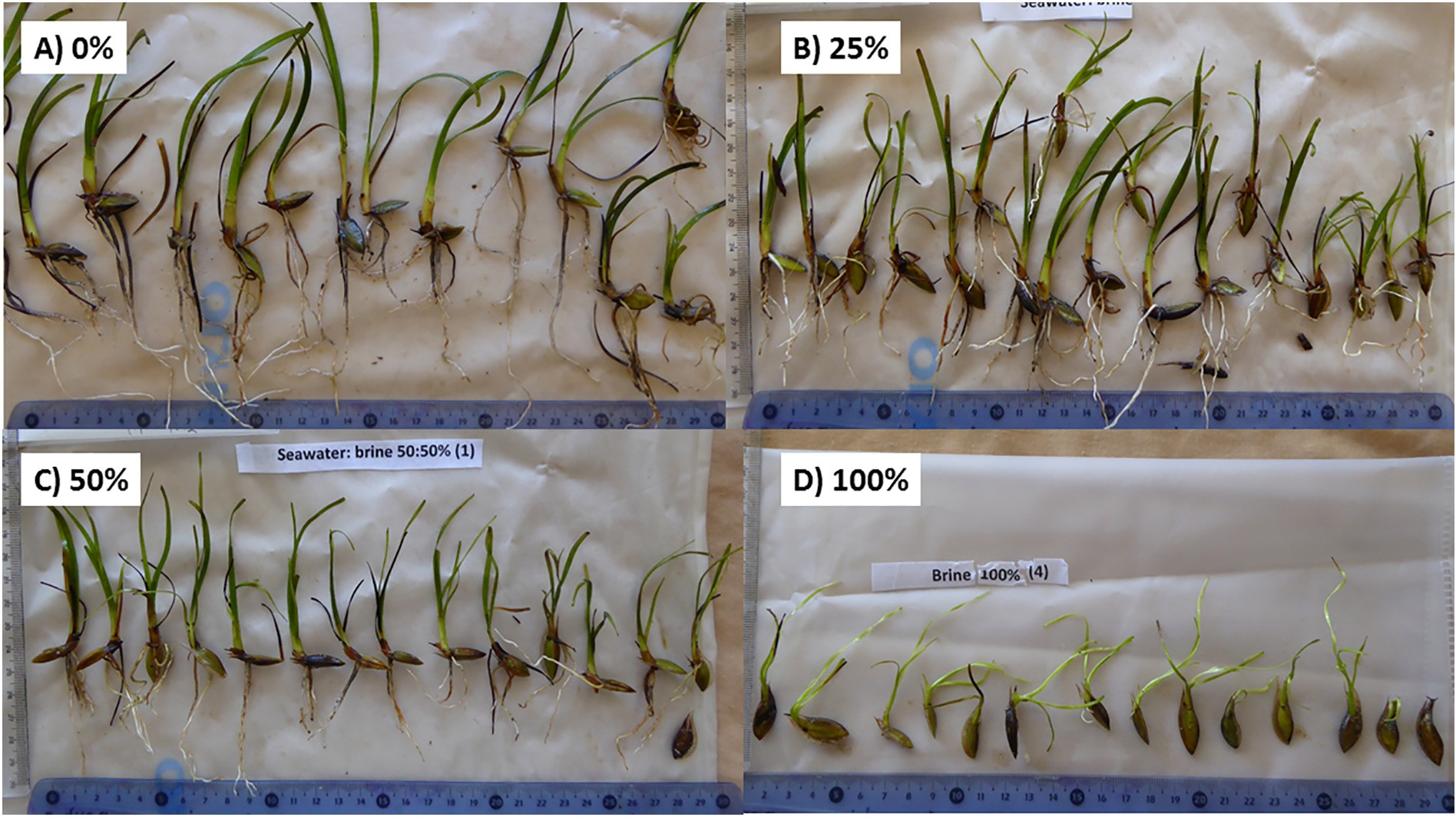
Fig. 2.12Picturization of seagrass Posidonia oceanica seedlings after 7 weeks exposure to brines (0, 25, 50 and 100%) showing reduced leaf shoot and rootgrowth.
Studies showed detrimental consequences for seagrass, including increased morbidity and mortality, decreased root development, highly negativewaterpotentials,anincreasein osmoregulators such as amino acids and sucrose, and adrop in potassium and calcium ion concentrations).
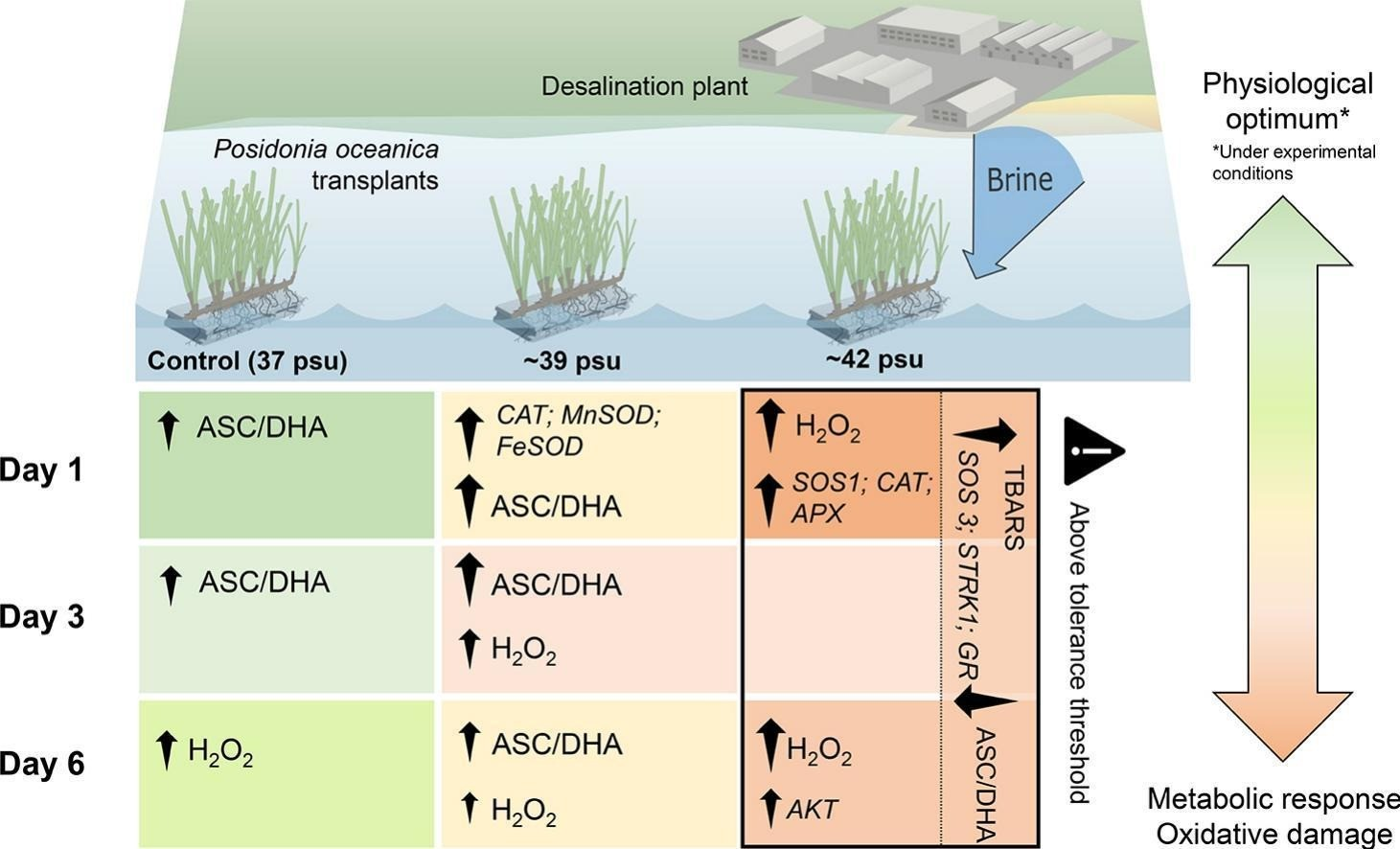
Fig. 2.13 Impact of brine on Posidoniaoceanica
In conclusion, small increments in salinity resulted in ~20?crease in shoot density, a large increase in leaf necrosis and reduced size and growth of surviving shoots, clearly all detrimental impacts on marine flora.
2.3.1.4?Effects on Diverse MarineFauna
Studies conducted on two fish species and a clam species were used to measure the effects of discharged desalination brine on marineorganisms. As the salinity increased from 45 ppt to 50 ppt, sea bream juveniles were affected within half anhour(bodycolorationdarkened)and the first death occurred within 24 hours, whereas juveniles exposed to 70 ppt died after one hour[35].
When the salinity reached 60 ppt, the hatching of flounder eggs slowed down, and no eggs hatched after the salinity reached 100 ppt.
Astudyonthe harmful toxicity of desalination brine on embryos and larvae of the euryhaline species, the Japanese medaka reported that as salinity increased to more than 35 ppt, the swim bladder inflation of the larvae decreased, incapacitating the fish and forcing them to consume more oxygen than larvae with inflated swim bladders. Furthermore, when exposed to salinities greater than 35 ppt, the eggs took much longer to hatch, reducing larval survival and competitiveness in the wild.
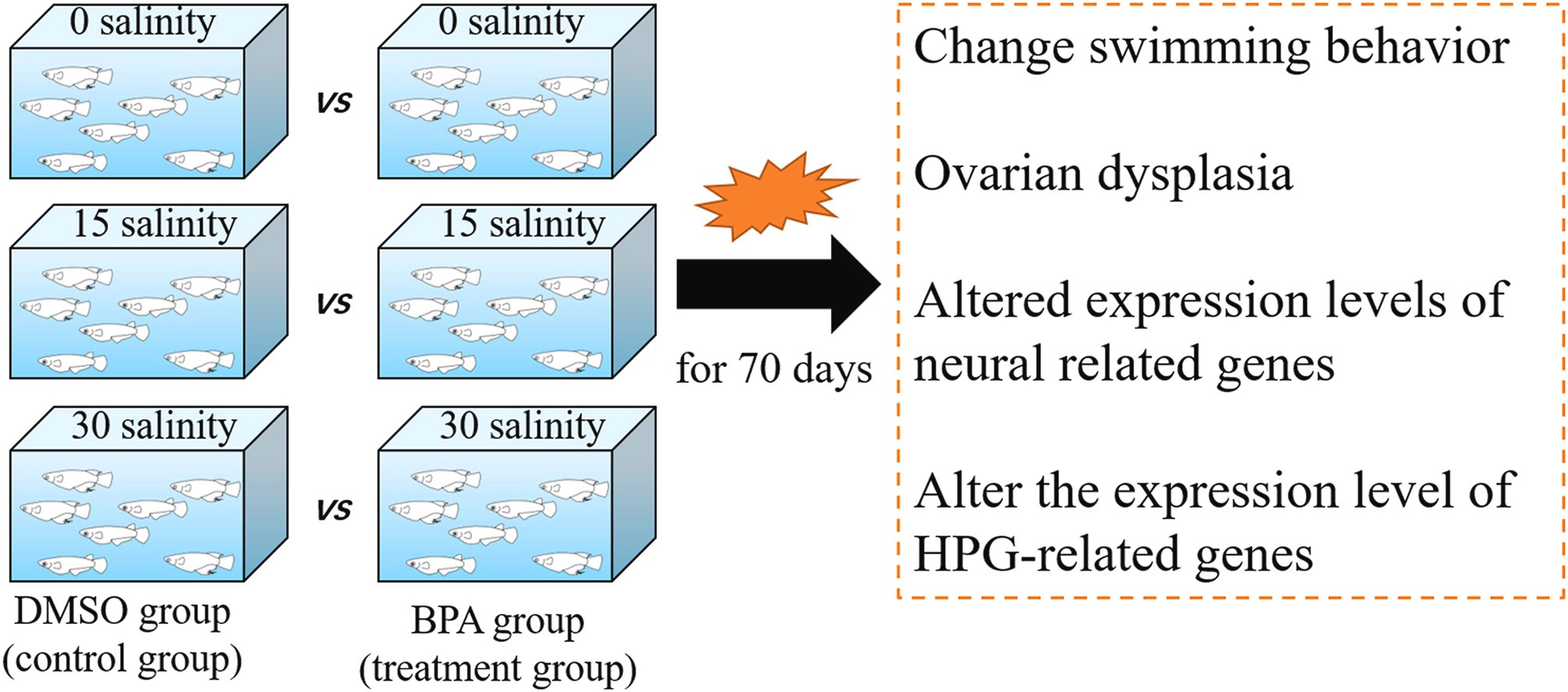
Fig.2.14Representationofastudyshowingtheeffectof increasing salinity on the Japanese medaka
Increased salinity also reduces gas diffusion in the eggs, resulting in smaller eggs and decreased hatching survival. Pathogenic infections also rise with increased salinity. Another effect of desalination brine is the concentration of metals and trace elements, which can causemortalityas metals prevent embryos from hatching. Elevated levels of magnesium reduce the activity of newly hatched eggs, inhibiting their movement and defensive mechanisms; and because brine is negatively buoyant and cuttlefish spend a part of their life cycle as benthic organisms, especially as benthic eggs, they are extremely vulnerable to bacterial infections.
Brine discharges have resulted in the depletion of fishpopulationsaswell as the death of corals and plankton in the Red Sea, while the Ras Hunjurah lagoon in the UAE suffered increased mortalityofitsmangroves and marine angiosperm, as well as increased pollution caused byinflated copper and nickel levels as a result of brine discharges.
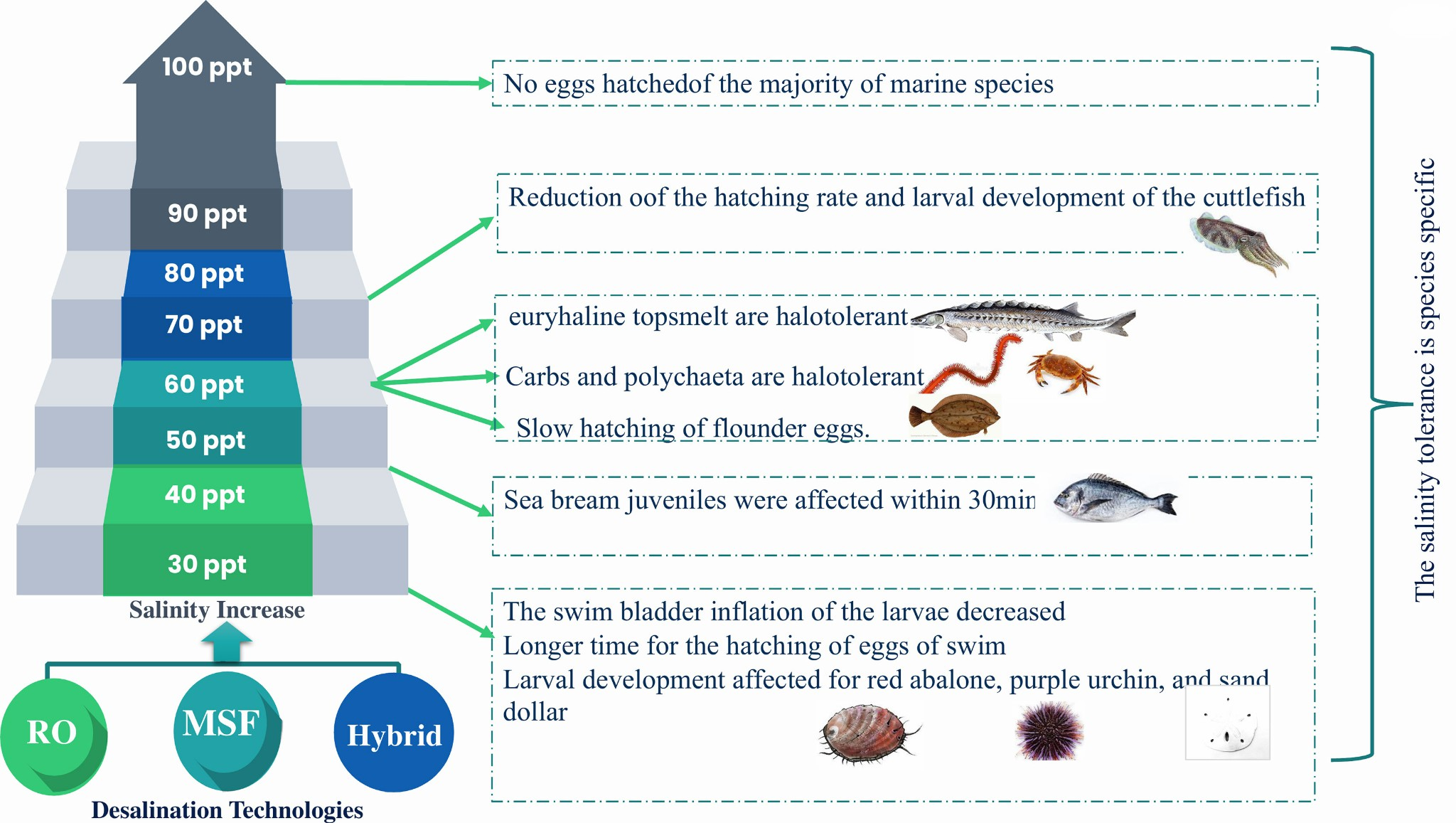
Fig.2.15Potentialimpacts ofbrines salinityon fauna(vertebrate andinvertebrate).
From the above case studies, it is clear that changes in salinity can adversely affect marine life, ranging from species depletion, change in behaviour, limited reproduction- reducing their overall survival rate in the aquatic environment.
2.3.2.?Effects on HumanLife
Unsafebrinedischargebydesalinationplantscanhaveseriouseffectsonhuman life due to the accumulation of toxic ions such as Strontium, Chloride and Sodium. The site for the desalination plants should be carefully selectedand made sure to be away from residential areas particularly forforwardplanningfor possiblefutureexpansions.Inmoderntimesthedesalinationcapacitysignificantly increased from approximately 35 million m3daily(MCM/day)in2005toabout95 MCM/day in 2018. Seawater desalination accounts for about two-thirds ofglobal desalinationcapacity,whilebrackishwaterdesalinationaccountsfortherest.
Membrane desalination, mainly using reverse osmosis (RO), is the primary desalination technique practiced globally, with MSF and MED following its lead. Despite the undeniable role of desalination for securing water supply in areas where natural freshwater supplies are scarce, desalination impacts the natural environment at different aspects. The concerning issues noticed are noise pollution, visual pollution, reduction in recreational fishing and swimming areas, emission of materials into the atmosphere and, the disposal of brine waste are the main cause ofpollution.[36]Aplethoraoffactorscontributingtopollutionhas highly impacted human health and the environment. Brine waste also poses a potential threat to water quality, as it contains alarmingly high concentrations of salts and otherminerals.Becauseofitshighdensityandsalinity,brinewastecan accumulate in and around disposal areas smothering bottom dwelling species and significantly altering coastal ecosystems, this can have indirect and severe effects on the human health through consumption of marinelife.Brinedeposited water bodies cannot be effectively reused for other purposes due to the vicious cycle of environment friendly brine water disposal methods being trapped. Although desalination provides several benefits to the human population, the severe repercussions associated with the disposal of industrial brine waste, ultimately calls for more sustainable and environment friendly ways to produce fresh water. [37]
2.4?PRODUCTION OF BIOMETHANE FROM ROCONCENTRATE
Chapter 2.1 detailed the process of RO desalination in theUAE,itsimportanceandthe techniques of brine disposal obtained from these various plants.On the contrary, the following chapter (Chapter 2.2) highlighted the negative impacts of the disposal of this brine concentrate on the marine ecosystem and well as human life. This chapterfinally addresses the crux of this study; the production of renewable biomethane from brine waste. The theoretical model proposed (as shown inFig.2.5),canservedualpurposes namelyreducingtheimpactofsurface water discharge of brine and reducing emissions
and in turn the massive carbon footprint of these desalination plants. The promotion of more renewable sources of energy such as Biomethane, is in accordance withSDG 7. This SDG primarily focuses on providing access to clean and cost effective modes of energy to under-developed and developing countries.The solution we provide below involves the production of a renewable source of energy that can be easily obtained and distributed. Recycling waste products is a cost effective and sustainable method of providing energy, and can positively impact the population, the economy, and the environment. It addresses the detrimental effects of the overuse of non-renewable resources such as coal,petroleumandnaturalgas,whichareestimated to be depleted completely by the year 2060.
Biomethane is a fuel that can beproducednaturallybytheprocessofmethanogenesis. This process has already been used for human advantage by biogas production plants that utilise acetoclastic methanogens extracted from the rumen of ruminant animals suchascattle,sheepandgoats.Howeverthisexactsametechniquecannotbeusedfor our model as the optimum growth conditions of these methanogens doesnt include a highly saline environment such as the brine concentrate that we wish to use for the process.
This issue can be resolved by using species from various genera of family Methanosarcinaceae; namelyMethanohalophilus and Methanococcoidesboth of which are characterised by the presence of numerous adaptations to overcome hypersaline stress as well as various adaptable pathways of methanogenesis.
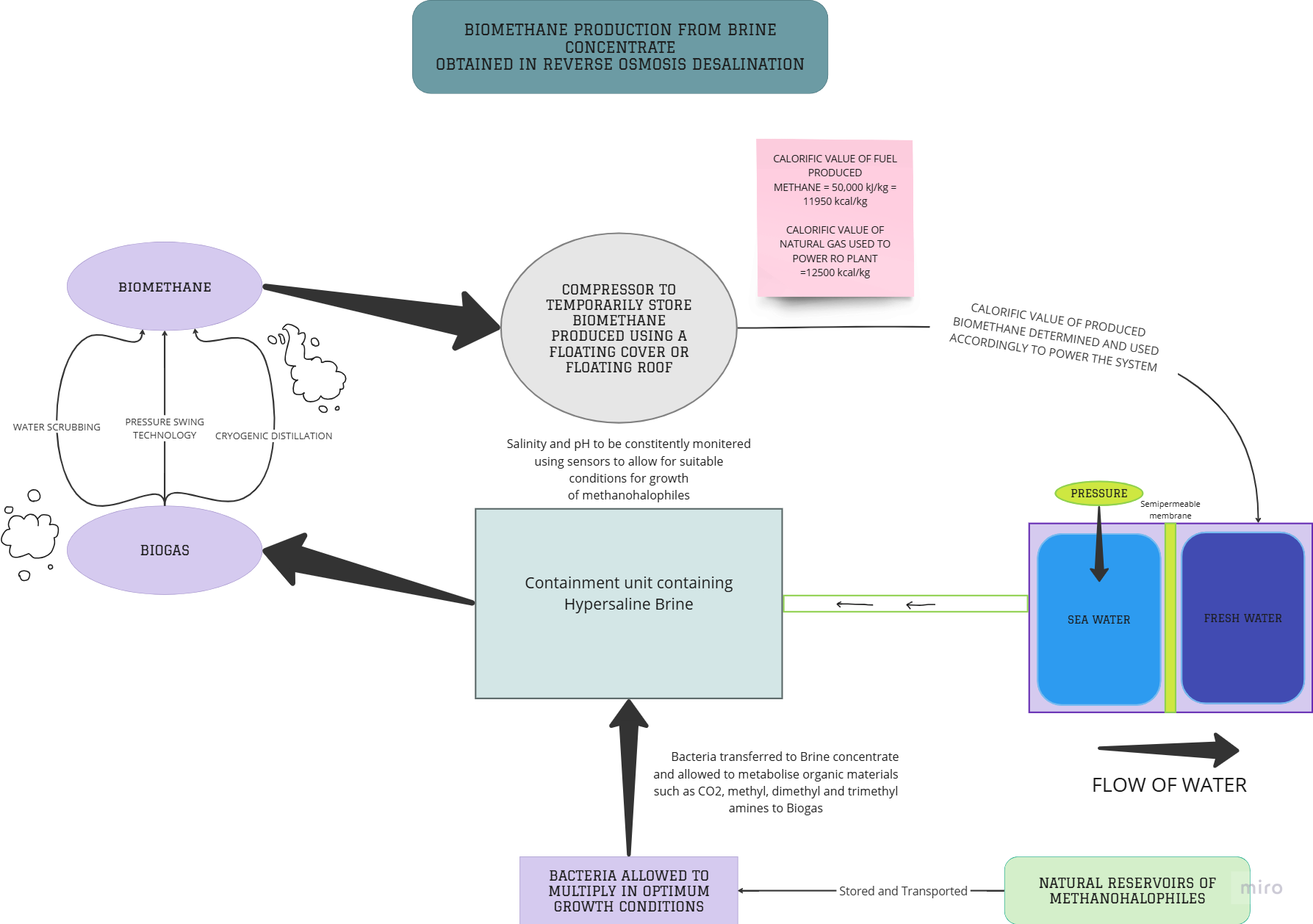
Fig. 2.16Flowchart illustrating the steps of the proposedmodel
- ?Adaptations and Characterisations of GenusMethanohalophilus
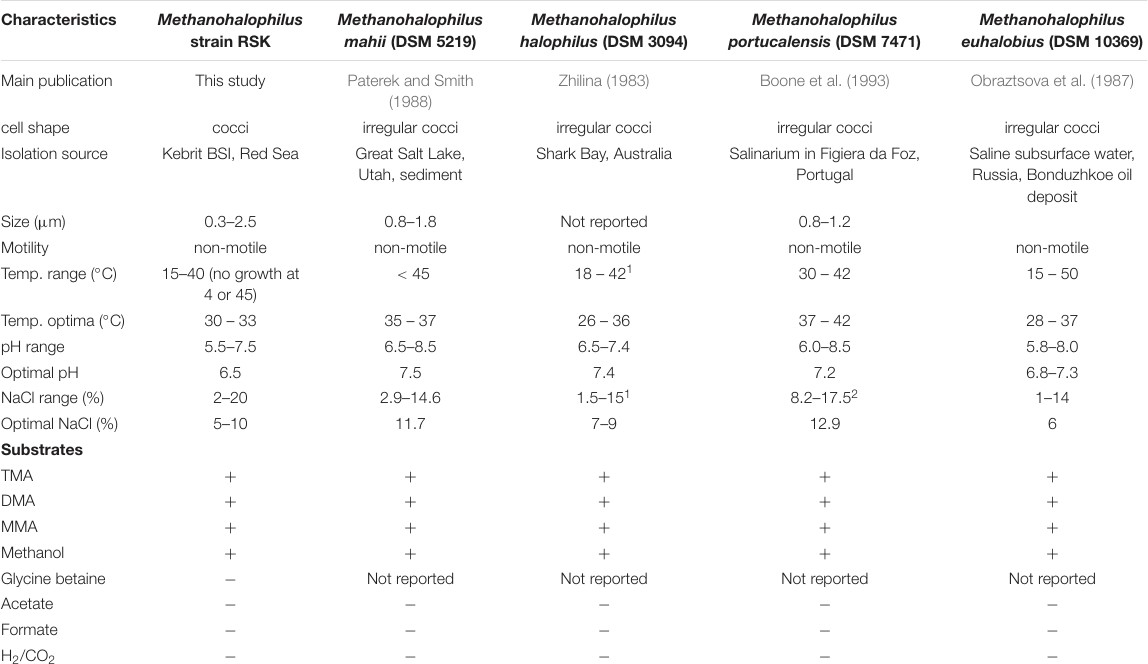
The genomes of all the species inmethanohalophilusindicate that it codes for thecompletesetofenzymesinvolvedinmethylotrophicmethanogenesisbutlack the enzymes required for the hydrogenotrophic pathway that can reduce H2,CO2and formate into methane.Methanohalophilushas various adaptations and strategies that confer its resistance to hypersaline and anoxic conditions of its native environment. These adaptations include
?OxidativeAdaptations
The strains of methanohalophilus which are referenced in this study were commonly isolated from brine-seawater interfaces, where the amount of dissolved oxygen is found in very low quantities. Therefore, it follows that these organisms must have certain adaptations to ensure that they can tolerate some amount of oxidative stress. Accordingly, the genomes of strain RSK and type strains Methanohalophilus encode up toeightcopies of thioredoxin, up to six copies of different isozymes of catalase peroxidase,peroxiredoxin,alkylhydroperoxidereductase,and alkyl-hydroperoxidase, and three copies of rubrerythrin. Thioredoxin reductase, catalase,andsuperoxidereductasewerealsodetectedineach genome. Among the above mentioned enzymes, thioredoxin reductase and thioredoxintogetherformthethioredoxinsystemwhichplayacardinal role preventing oxidative stress in providing thioredoxin based oxidative redox regulation.
?OsmoticAdaptations
The hypersaline environment of these organisms naturally calls for mechanisms to maintain theosmoticbalanceoftheircells.Allcellshavea certain osmotic pressure which they must maintain by the selective permeation of ions that confers said osmotic pressure.
Species in the genusMethanohalophilusprimarily follow a salt instrategy, which involves the intracellular accumulation of K+ and Cl- ions with the help of protein pumps present in the cell membrane.
The presence of a K /Na symporter Ktr along with the multiple copies of the potassium uptake system Trk and the glycine betaine/L-proline transport system permease (ProW) support this method of maintaining osmotic balance. [41]
2.4.1.2Methanohalophilus mahiisp.nov
- Genus : archaea, Methanohalophilus
- Family : Methanosarcina Care within the order
- Strain - strain SLP (Salt Lake Paterek). This strain has been deposited in the American Type Culture Collection as strain ATCC
- Unique feature - These microorganisms are notable for their ability to survive in extremely saline (salty) environments, as well as their ability to produce methane.
- Members of this genus are non spore forming, nonmotile, andoccur singly or in small clumps.
- The colonies fluoresce yellowish green under 420-nm
- Source :this strain is isolated anaerobic sediment from GreatSalt Lake, Utah.
- This methanogenesis requires : sodium, magnesium, iron, and potassium, maximum response occurs in sodium concentration between 0.5 and 2.5 M.
- Temperature optimum : 35C, no growth at temperatures above 45C.
- pH optimum : approximately 5
- Physiology :fastidious
- Substrate: Trimethylamine, dimethylamine, methylamine, and methanol serve as asubstrate for growth and methane production.
Methanohalophilus mahiiis a unique organism and not a marine methanogen which has adapted to a hypersalineenvironment.The microorganism requires sodium chloride concentrations in the moderately halophilic range of 1.0 to 2.5 M for optimal growth and methanogenesis. Methanol and methylamines are the substrates that are utilized for growth.M. mahiiis isolated from thesediments of the Great Salt Lake. The optimum temperature and salinity for growth and methanogenesis were 37C and 2.0 M NaCI, respectively.
2.4.1.2?Methanohalophilus zhilinae sp.nov
- Strain-WeN5tstrain,
- Source-ThestrainwasisolatedfromBosaLakeinWadiel Natrun, Egypt
- Genus - Methanohalophilus
- Salinity-Thelakewaterwasalkaline(pH7)andsaline,withhigh total dissolved solids content (245 g/L)
- Substrate -
- Itcangrowonmethylamines(dimethylamine, trimethylamine) and methanol.
- Atlowconcentrationitutilisesdimethylsulfideasacatabolic
- Growth rate -
- grows in both complex and mineral
- yeast extract ,trypticase peptone increases growth rate
- Temperatureoptimum-45C
- pH optimum - 9.2 (approx)
- Requirement-
- high concentration of NaCl and other salts such as calcium .
- Methanogenesiswassensitivetohighconcentrationsof sulphide. cultured strain with minimum concentration of sulphide exhibited delayed or no methane production.
- StrainWeN5Tshowedresistancetocellwallsynthesisinhibitors (penicillin G, ampicillin)
Methanohalophilus zhilinae, a new alkaliphilic, halophilic, methylotrophic species of methanogenic bacteria, is described. Strain WeN5T (T = type strain) from Bosa Lake of the Wadi el Natrun in Egypt was designated the type strain and was further characterized. This strain was nonmotile, able to catabolize dimethylsulfide,andabletogrowinmediumwithamethyl group-containing substrate (such as methanol or trimethylamine)
TableVIprovidesanoverviewofthecomparativegenomicsbetweenthedifferentstrains in genusMethanohalophilus[38]
TableVICharacteristicsofdifferentstrainsandspeciesingenusMethanohalophilus
2.4.1.3?Case study: Lake Elton saline aquaticsystem
- Method-Cultivationandmolecularapproacheswereusedtostudy methanogenesis in saline aquatic system
- Source-sedimentsinthesalineaquaticsystemofLakeElton (southern Russia), the largest hypersaline lake in Europe.
- SalinityRange:7to275g/L.
- The methylotrophic route showed activityacrossallsalinitylevels, with Methanohalobium and Methanohalophilus as the genera in fully saturated salt conditions while Methanolobus and Methanococcoides exhibited moderate halophilicity, in lower salinity environments.
- No methane is produced from formate, acetate, and H2/CO2 in hypersaline condition due to high energy limitations.
- Temperature-Under mesophilic conditions, hydrogenotrophic sulfate-reducing bacteria (SRB) and methylotrophic methanogens outcompete methylotrophic methanogens, while under thermophilic conditions it is the opposite .
- Thecontributionofhydrogenotrophic,acetoclastic,and methyl-reducing methanogens to the community increases with a decrease in salinity.
- Methane Emission-In hypersaline environments the combination of methane production and minimal methane oxidation could lead to the release of methane into the atmosphere.
Cultivationandmolecularapproacheswereusedtostudymethanogenesisinthe saline aquatic system of Lake Elton (southern Russia), the largest hypersaline lake in Europe. The most active pathway detected at all salinities was methylotrophicwithadominanceofMethanolHalobiumandMethanohalophilusgenera,atsaltsaturationandmoderatelyhalophilicMethanolobusand
Methanococcoidesat lowersalinity.Anactivemethaneproductiontogetherwith negligiblemethaneoxidationmakeshypersalineenvironmentsapotentialsource of methane emission
- ?Adaptations and Characterisations of GenusMethanococcoides
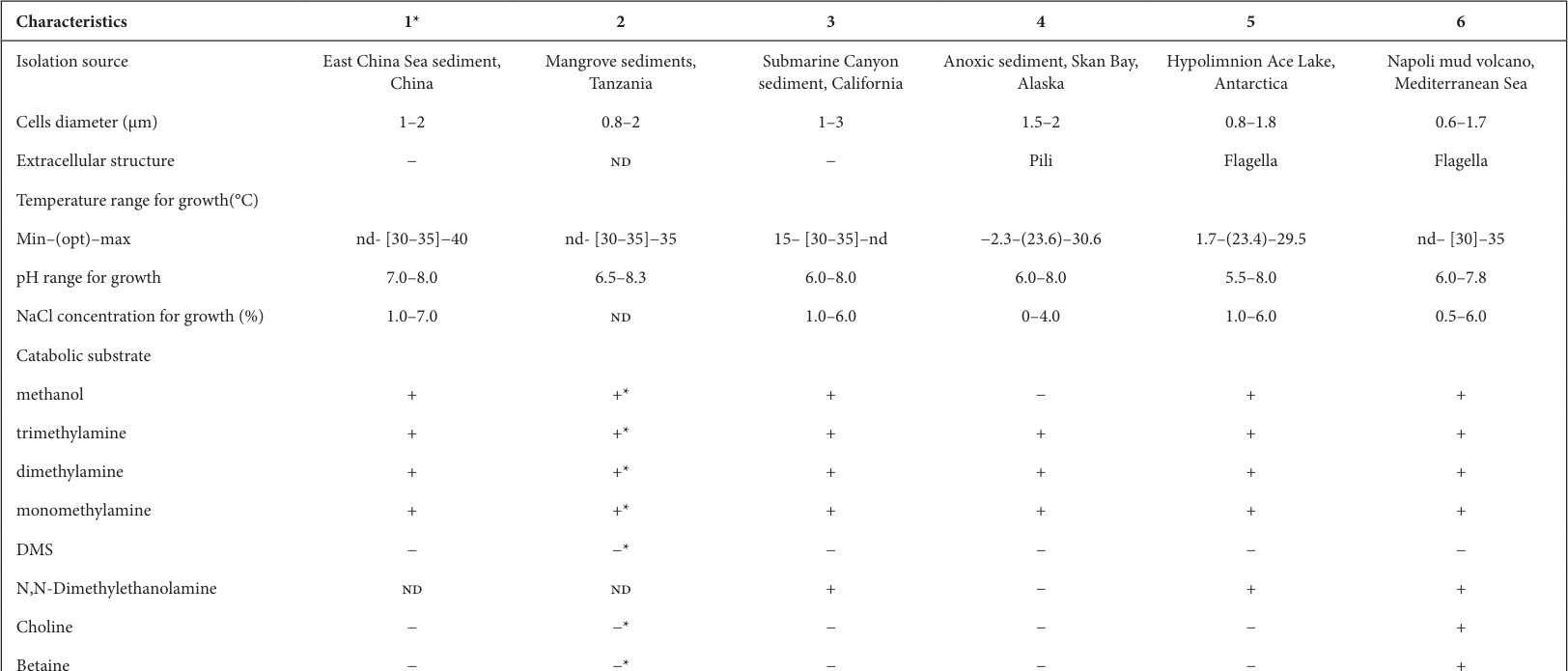
The genus Methanococcoides belongs to phylum Euryarchaeota , class Methanomicrobia, order Methanosarcinales, and family Methanosarcinaceae. It was first described by Sowers and Ferry for irregular cocci methanogenic archaea utilizing methylated compounds like methanol, methylamine, dimethylamine, trimethyl amine as substrates for growth and methanogenesis.
2.4.2.1?Methanococcoidesmethylutens
- Strain- TMA-10
- Source-ThestrainwasisolatedfromBosaLakeinWadiel Natrun, Egypt
- Genus - Methanococcoides
- Salinity -5 - 6.0 v/v% of NaCl
- pH optimum - 7.0 - 5
- Substrate -
- ItutilisesTrimethylamine,diethylamine,monomethylamine, and methanol as substrates for methanogenesis
- Cangrowmixitrophicallybyundergoinghydrogenotrophic methanogenesis with DIC (Dissolved Inorganic Carbon)
- Growth rate -
-Growth on trimethylamine was stimulated by yeast extract, Trypticase(BBLMicrobiologySystems,Cockeysville,Md.), rumen fluid, or B vitamins.
- Temperatureoptimum-30-35C
- pH optimum - 9.2 (approx)
- Requirement-
- HighconcentrationofNaclandothersaltssuchasMgSO4are required for growth.
Methanococcoidesmethylutensisamethylotrophicmarinemethanogen,thetype species of its genus. It utilisestrimethylamine,diethylamine,monomethylamine,
andmethanolas substrates for growth andmethanogenesis. Cells are
non-motile,non-spore-forming,irregularcocci1?mindiameterwhichstainGram-negativeandoccursinglyorinpairs.TMA-10isthetypestrain(ATCC 33938). [39]
TableVIIsummarisestheoptimumgrowthconditionsandsubstratesusedbydifferent species in the genusMethanococcoides.[40]
Table VIIDescriptive and catabolic features of species in genus Methanococcoides Strain/species
- Methanococcoides orientis LMO-1T (=MCCC 4K00106T=JCM 39195T) 2. Methanococcoides methylutens MM1 (DSM 16625) 3. Methanococcoides methylutens TMA-10T (DSM 2657T) 4. Methanococcoides alaskense AK-5T (DSM17273T)5.MethanococcoidesburtoniiACE-MT(DSM6242T)
- Methanococcoides vulcani SLH33T (DSM26966T).
2.4.3?Metabolic pathways and energetics ofmethanogenesis
There are 4 known metabolic pathways of methanogenesis to date, namely Acetoclastic (utilising acetate), Hydrogenotrophic (Utilising H2/CO2), Methylotrophic (Utilising substituted methanes such as Methanol, DMA (Dimethylamine) and TMA (Trimethylamine). In hypersaline environments methylotrophic methanogenesis is the primary metabolic pathway, and often accompanied by hydrogenotrophic metabolism as well. [41]
This report details extensive studies on formation of methane via two of the threemainpathwaysinmethanogenicarchaeahasbeenstudiedintensively.For example, mixotrophically growing cultures of Methanosarcinabarkeriformtheir biomass equally from methanol and CO2; however, almost all the methane is formed frommethanolratherthanfromCO2sincemethanolisdisproportionated to methane and CO2 according to the following reaction:
Halophilic methanogens contribute signi?cantly to carbon mineralization in marine and hypersaline environments. The preferred substrates for methanogenesis in these habitats are C-1 methylated compounds, for example, methylaminesthatareconstantlyprovidedbythedegradationofosmolyteslike
glycine betaine or membrane compounds such as choline. The preference of methylated C-1 compounds over hydrogen by methanogens thriving in saline environments re?ects a competitionwithsulfate-reducingbacteriathatareable to utilize hydrogen more e?ciently than methanogens, but usually cannot use methanol or methylamines as substrates.
2.4.3.1?Methylotrophicpathway
The methylotrophic pathway is the dominantly observed pathway in marine and hypersaline environments. Methyl-based methanogenesis includes methanogenesis from the following methylated compounds: tetramethylammonium, trimethylamine (TMA), dimethylamine (DMA), monomethylamine (MMA), methanol (MeOH), glycine betaine (GB), dimethylsul?de (DMS), methanethiol (MT), and methylthiopropanoate (MMPA).
Fig. 2.17 illustrates the various chemical reactions that occur to produce methane from the methylated compounds mentioned above. The genes and enzymes involved in the process are also highlighted alongside their respective steps. [42]
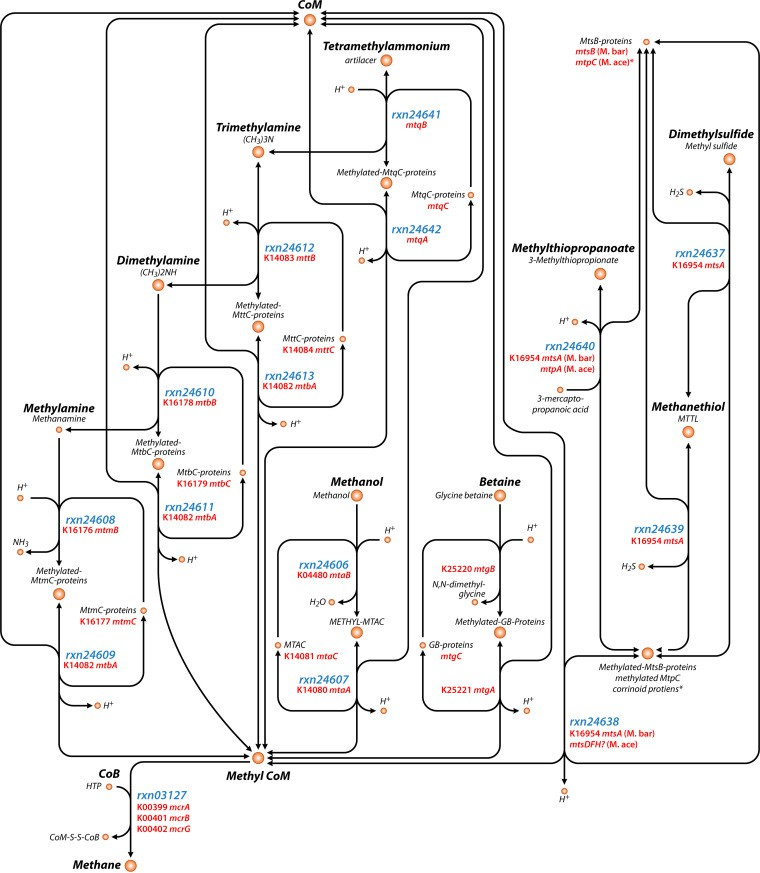
Fig. 2.17Variouspathwaysinvolvedinmethaneproductionfrommethylated compounds
Methyl-based methanogenesis can be the dominant methanogenesis pathway in anaerobic ocean environments (e.g., sediments and hydrothermal vents), coastal environments (e.g., seagrass meadows and coastal wetlands), other environments with high salt and/or sulfate concentrations (e.g., solar salterns, hypersaline lakes, and microbialmats), and even insect guts. This is because some methylotrophic methanogens such as Methanolobus, Methanosalsum, Methanohalophilus, Methanohalobium, and a potential new Methanosarcinaceae genus identified by metagenomics, are halotolerant or halophilic organisms; while they group phylogenetically with other methanogens, they contain the biochemical mechanisms necessary to cope with high salt via production of compatible solutes or by having an acidic proteome. Methyl-based methanogens are also favored in saline environments because the compatible solute glycine betaine producedby halophilic bacteria is fermented to TMA or used directly, which fuels methyl-basedmethanogens.Anotherpotentialreasonwhythesemethyl-based methanogenic taxa survive in hypersaline environments while othertypesofmethanogensdonotisthattherelativeenergyyieldof the methyl-based pathway is higher, which enables them to divert energy to compatible solute production [43].
2.4.3.1?Hydrogenotrophicpathway
Hydrogenotrophic methanogenesis is believed to be the most ancestral form of methanogenesis based on phylogenetic and proteomic studies. Class I methanogens (Methanopyrales, Methanococcales, and Methanobacteriales), as well as most class II methanogens (Methanomicrobiales, Methanocellales, and Methanosarcinales) are hydrogenotrophs.
They reduce CO2to CH4in six steps via the reductive acetyl-CoA or WoodLjungdahlpathway(WLP).TheWLPisoneofthemostimportant
processes for energy generation and carbon fixation. Here, H2, or sometimes formate, is used as an electron donor. Hydrogenotrophscouple the WLP to methanogenesis to conserve energy. This coupling is established by N5-methyl-tetrahydromethanopterin:coenzyme M methyltransferase(Mtr;alsoknownastetrahydromethanopterin S-methyltransferase), which transfers the methyl group from the WLP to coenzyme M. Mtr uses the free energy of methyl transfer to establish a Na+-motive force across the membrane. Methyl coenzyme M reductase then reduces methyl-coenzyme M to methane, using coenzyme B as an electron donor. The established disulfide bond between thesecoenzymes is then broken again by heterodisulfide reductase (drABC/mva DG). This cytoplasmic electron bifurcating complex concomitantly generates the reduced ferredoxin required for CO2 reduction in the process.
Fig 2.18 explains these steps involved with great detail and provides insightonthebiochemicalmechanismsinvolvedinthecomplexprocessof hydrogenotrophic methanogenesis [44]
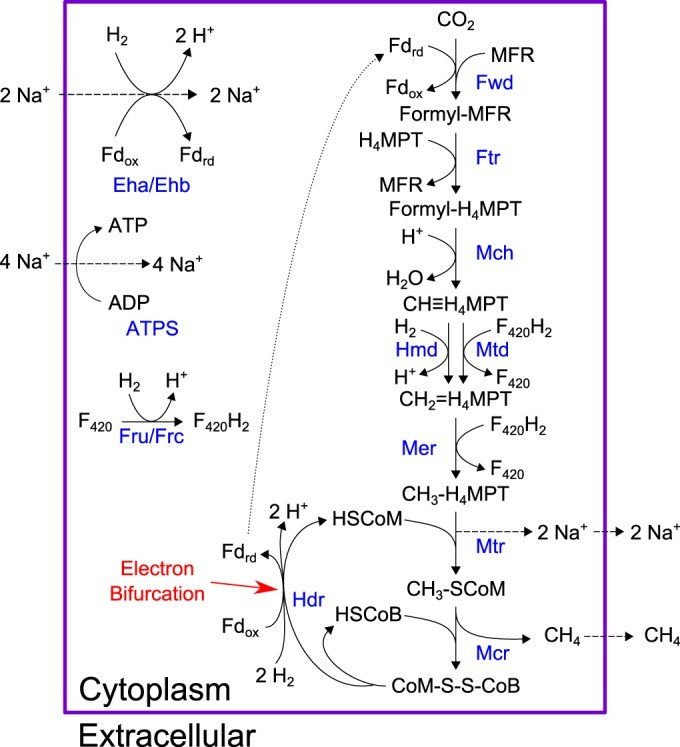
Fig. 2.18Steps involved in hydrogenotrophicmethanogenesis
2.5?STORAGEANDUSEOFBIOMETHANE
Storage of biomethane through different means include: integration into the gas grid, underground reservoirs, liquefied forms, and bottling. Important emerging technologies in biomethane storage are: absorbed storage using metal-organic frameworks(MOFs) andotherporousmaterials,aswellasphysicalandchemicalconversionsintoclathrate hydrates and chemicals. We can enhance our biomethane storage capabilities by making a broad infrastructure strategy.
Biomethanecanbestoredinacompressedformunderpressureascompressed biomethane (CBG).
2.5.1?Storage ofBiomethane
Biomethane is less corrosive than biogas and has greater potential value as a fuel.Asaresult,storingbiomethaneforon-oroff-farmuseisbothfeasibleand advantageous.
2.5.1.1?High-Pressure Storage of Compressed BiomethaneCompressed Biomethane (CBM) optimizes the spatial setup for putting awaybiomethane.Theoperationofgasscouringattallweightconditions is pivotal to dodge the condensation of debasements such as H2S or
water that causes corrosion.
Compressinggasestoveryhighcompressivepressures(from2,000upto 5,000 psi) is considerably more expensive than compressing them at medium pressures. This is why biogas upgrading to more valuable biomethane usually occurs before compression. The energy required to compress to 2,000 psi is approximately 14 kWh per 1,000 cubic feet of biomethane, assuming biogas is upgraded to 97% methane with a heat rate of 12,000 Btu/kWh. Therefore, energy would amount to 17% of gas's energy content in the case of compression, in this case.
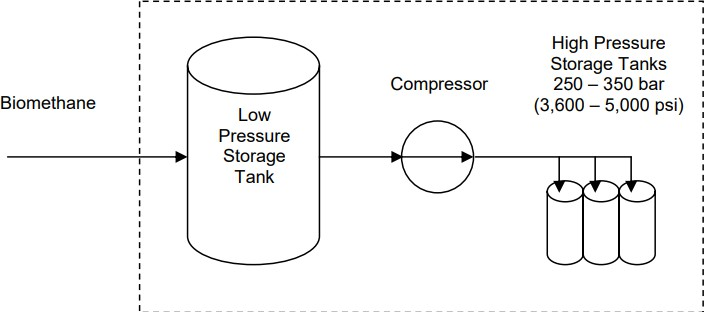
Fig.2.19Schematicofon-farmstoragesystemforcompressedbiomethane
Alow-pressurestoragetankactsasabufferfortheoutputfromthebiogas upgrading equipment, typically consisting of one or two large, airtight vessels with a storage capacity of one to two days' worth of biogas production.Forinstance,adairywith1,000cowsproducesroughly30,000 cubic feet of biomethane per day. Slight compression of the biomethane increases the gas volume stored in the low-pressure tank. Large, stationary low-pressure storage tanks are custom-designed and available from various manufacturers.
Given the unlikely scenario of sufficient on-farm vehicle demand for all producedbiomethane,mostofitmustbetransportedtorefuelingstations. Due to its low energy density at atmospheric pressure, the most economical and efficient transportation method for upgraded biogas is in compressed form. With CNG refueling stations providing CNG at 3,000 to 3,600 psi, CBM should be transported at similar or higher pressures to minimize additional compression at the refueling station.
The compressor takes low-pressure biomethane from the storage tank andcompressesitto3,600to5,000psi.Thecompressormustmatchthe
output flow rate from the biogas upgrading equipment, such as 2,000 cubic feet of raw biogas per hour for a dairy with 1,000 cows. Several manufacturersprovidesuitablecompressors,likeBauerCompressorsand GreenField Compression.
The CBM output is then fed to high-pressure storage tanks connected in parallel, housed in a portable trailer. For on-farm CBM refueling, these high-pressuretankscouldbestationaryandlarger.Portablehigh-pressure storage tanks for this application are available from manufacturers like Dynetek Industries and General Dynamics.
2.5.1.2?Storage of liquified ofbiomethane
Liquefiedbiomethane(LBM)offersseveraladvantages,includingeaseof transportation and compatibility with both LNG and CNG vehicles. To transport LBM off-farm, it must be stored in tanker trucks with a
10,000-galloncapacity,requiringon-farmstorageuntilthisvolumeis reached.
LBM storage involves low-pressure tanks that serve as buffers after biomethane exits liquefaction equipment. Typical LNG storage tanks are double-walled and thermally insulated, with capacities of 15,000 gallons for aboveground applications. For smaller needs, 6,000-gallon tanks are available,thoughon-farmdemandforLBMislikelylow.Adairywith1,000 cows would produce about 15,000 gallons of LBM in six weeks. The LBM is stored at around 50 psi in these tanks, which cost approximately
$170,000 each from suppliers like NexGen Fueling.
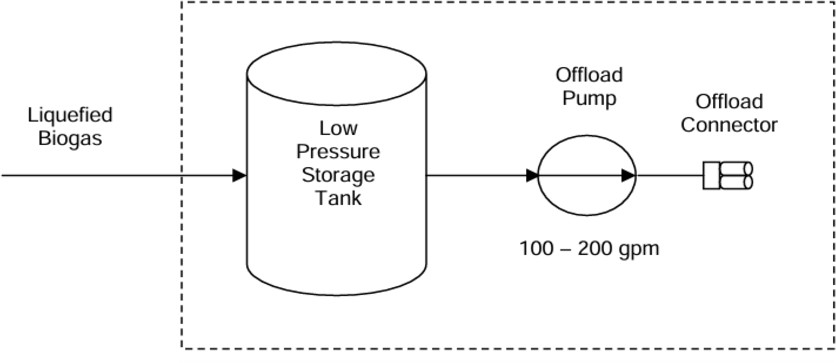
Fig 2.20Schematic to show storage of liquidBiomethane
Giventhelowon-farmvehicledemand,mostLBMmustbetransportedto refueling stations for natural gas vehicles. LBM is transported similarly to LNG, using insulated tanker trucks designed for cryogenic liquids.
Standard trucks hold 10,000 gallons at around 50 psi. An offload pump, withtypicalflowratesof100to200gallonsperminute,isusedtotransfer LBM from storage tanks to the tanker. These pumps, costing between
$15,000 and $25,000, include standard LNG connectors. [45]
2.5.2?Using Biomethane to Power the ROPlant
In Fig.2.18, it can beobservedthatonemoleculeofCO2intheHydrogenotrophic pathway of the halophilic methanogens with methanol, one molecule of CH4is produced. This cycle can be repeated as long as the salinity of the brine is favourable to the optimum growth conditions ofthemicroorganisms.Similarly,as referenced in section 2.3.3 of discussion, 4 molecules of CH3OH can produce 3 moleculesofCH4.Henceacolonyofhalophilicmethanogenscanproducealarge amount of biomethane.
The calorific value of a fuel is the total energy released as heat when asubstance undergoes complete combustion with oxygen under standard conditions. Biomethane hasanetcalorificvalueof36MJ/m3,whichisequivalent to 10 kWh/m3. In a reverse osmosis plant, 3 to 10 kWh of electric energy are typically needed to produce one cubic metre of freshwater from seawater. Therefore the energy required to power the RO system lies within the range of the energy provided by the halophilic methanogens.
- ?RESULTSANDCONCLUSIONS
The results of the discussion yielded significant revelations and contributed to the development of the theoretical model displayed in Fig 2.13.ThecomplexissueofBrine concentrate disposal into oceans has been tackled and the solutions provided aim to mitigate the harmful consequences of this disposal.
There were certain calculations used to relate the salinities of the RO plants with the optimum salinities of the species that were illustrated in Table VI and Table VII.
The salinity from ppm was first converted to mol/L using the formula:

Molar mass of NaCl = 58.44 g/mol
The value in mol/L was then converted to V/V% using the formula:
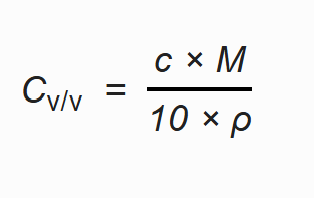
Where C is concentration in mol/L
M is molar mass of substance
?isdensityofthesolution
The density of brine usually varies from 1.02 - 1.05 g/cm3[46]
Table showsatabularrepresentationofthedatacollectedandcalculatedfromthis formula
TableVIIISalinityof11ROplantsacrosstheUAEinppm,mol/Land%v/v
The organisms mentioned in Chapter 2 are versatile in both metabolic and adaptation wise, and therefore allow for a wide range of possiblemodelstoproducethesameend product i.e Biomethane. The organic compounds (TMA, DMA, Methanol) as well asCO2 present naturally in brine will act as substrates to facilitate the process. Currently there are no studies done to calculate the exact values of these compounds, so it is necessary for more information on the topic to close the research gap regarding our theoretical model. Fig 2.16isalinegraphthatshowstheV/V%ofNaClin11ROplants from the UAE and the average of the optimum NaCl concentration of organisms were plotted and matched with the salinities of the RO plants. The salinity in mol/L was also plotted to provide a graphical representation of the minor fluctuations in salinities between the plants discussed.
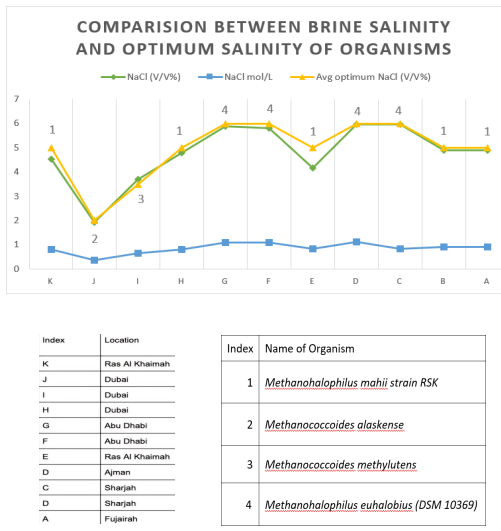
Fig.2.21Rectilineargraphcomparingthesalinityofbrineconcentratefrom11RO plantsin the UAE with optimum salinities of various Halophilic methanogens
CHAPTER 3:CONCLUSIONS
The following conclusions were drawn from the report:
3.1?DIFFERENT PRACTICES OFDESALINATION
There are mainly two categories of desalination technologies thatarepracticedtodate: thermal and filtration based desalination techniques. Thermal desalination is further categorized into: solar still, humidification-dehumidification and multistage flash distillation. Filtration based desalination includes forward osmosis, membrane distillation, electrodialysis desalination, capacitive deionization desalinationandreverse osmosis.
3.2?SEAWATERDESALINATIONINUAE:REVERSEOSMOSIS
- Reverse Osmosis is a process in which the feed water is provided with a pressure that is greater than its osmotic pressure and this is passed through a semipermeable membrane, thereby enabling onlyfreshwatertopassthroughthe membrane and rejecting the brine concentrate.
- More than 20 Seawater ReverseOsmosis(SWRO)plantsexistacrossThe major plants include:
- Brine disposal methods comprise of the following:
- Diluting the brine concentrate and then discharging it back into the
- Deep-Well Injection: In this method, the brine is injected deep underground into salt caverns or geological formations, isolating it from thesurfaceItisstillbeingexploredforlarge-scaleapplication in the UAE.
- Sprayirrigation:Brineisdilutedtobeusedforirrigationpurposesforthe
- Evaporation Ponds: This method is used to store saline solution and to reduceitsItisnotcurrentlybeingusedintheUAEduetoitscost as compared to disposal to sea.
- Utilisingdesalinatedwaterforirrigationcouldpotentiallydegradesoilstructure, due higher concentration of sodium and boron ions present in it.
3.3?IMPACTSOFBRINECONCENTRATEDISPOSAL
ToxicchemicalsandsaltsinSWRObrineleftoverfromdesalinationhaveanadverse impact on local marine ecosystems. Studies detailed in Chapter 2.3 observed that multiple marine species were affected by desalination plant outfall, especially:
- Phytoplanktons,whichshowedanoteworthydecreaseintheirpopulationdueto presence of Cu in brine, which inhibited photosynthesis, restricting the uptake and assimilation of nitrate and the uptake of silicate.
- Calcareousbenthicforaminifera,whichshowedasignificantdeathrateinstudies conducted near 3 desalination plant outfall sites.
- Posidoniaoceanicaplants,whichshowedsymptomsofnecrosis,reducedleaf growth, and increased mortality rate at higher salinity levels.
- Diverse species of fish like the Japanese medaka showed decreased rates of larvalsurvivalandovariandysplasia,reducingspeciessurvivalandreproduction
Accumulationoftoxinslikesodium,chlorideandsodiumcancausesevereskinandeye irritation and burns.Increasing amounts of strontium can interfere with the normal bone development. Unsafe methods of brine can cause different types of pollution such as visual,noise and water pollution.reduction in recreational fishing and swimming areas, emission of materials into the atmosphere .High density and salinity present on the
sea-bedsdangerouslyaffecttheunderwatercreatureswhichindirectlyhasinimical effects on the human health.
3.4?PRODUCTION OF BIOMETHANE FROM ROCONCENTRATE
The conclusions drawn from this chapter were vital to solving the research gap earlier mentioned in the previous chapters as it discusses the characteristic features and the metabolic pathways of the microorganisms from the Methanosarcinaceae family, which is essential to the theoretical model that we proposed in this study. The characteristic features of two generaMethanohalophilusandMethanococcoideswas described in detail and this information was later used in section 2.6 to match the microorganisms with the respective RO plant in which they would be used to produce biomethane. The metabolic pathways detailed were the methylotrophic and hydrogenotrophic pathways which both have a high yield of methane with respect to their substrates.
3.5?STORAGEANDUSEOFBIOMETHANE
This section describes the various existing methods that are used tostorebiomethane. Biomethane can be stored in a compressed gas form or in a liquified form. This information can be used to store and transport the biomethane that is produced by the process. The produced biomethane can then be used to power the RO plant as it has high calorific value as discussed in this section.
3.6?RESULTSANDCONCLUSIONS
This section played a major role for gathering data and enabling our report to be more statistically relevant, by detailing all the major calculations and the graphs that were plotted based on the data gathered.
CHAPTER 4:RECOMMENDATIONS
This chapter primarily focuses on the various solutions that can be provided to the problems mentioned in Chapter 2: Discussion. The list of recommendations include variousmethodsthatcantransformthebrineconcentratefromawastefulbyproductinto a valuable resource that can improve the entire process of desalination. The solutions mentioned will also be in alignment with the Sustainable Development Goals (SDG 2030) proposed by the UN as mentioned in Chapter 2: Discussion.
In order to combat the depleting water resources and increase the efficiency ofseawater desalination, the following measures are also suggested:
- MXene,arecentlyidentified2Dnanosheet,hasdrawninterestduetoitspotential application in the modification ofthin filmnanocomposite (TFN) membranes for the production of clean water. MXene-based sulfonated TFN(STFN) membranes have a crumpled texturewhichleadstoenhancedfluxandimprovedwettability, thereby optimizing desalination performance. They also have greater antifouling and chlorine resistance properties [47].
- In the pre- and post-treatment stages, differentmembrane unitscan be utilized, which will lower the energy demands and environmental impacts of the wastes produced. FO as a pretreatment unit before RO can reduce the energy consumption because it does not requirehydraulic pressureas inmicrofiltrationandultrafiltration. On the other hand, RO effluents can also be utilized as MD feed solution for higher water recovery, while waste heat can be exploited astemperature gradientto drive MD. Salinity gradientenergy can be harvested by RED from brines of RO. This additional energy can partially compensate for the totalenergyneededforthedesalinationAlso,duetodilutionofretentatestreams, the environmental effect of brine disposal can be minimized [55].
- Arecentmethodcalledtemperatureswingsolventextraction(TSSE)isaradically different desalination technology that is membrane-less and not based on evaporativephaseTSSEutilizeslow-temperatureheatandalow-polarity solvent with temperature-dependent water solubility for the selective extraction of water over salt from saline feeds [48]
- CO2can be bubbled into ammoniated brinetoprecipitatesodiumions.Thebrine which leaves the RO membranes can be highly pressurized and mixed with an absorbent. The absorbed CO2 precipitates the sodium dissolved in the form of sodium bicarbonate, which can be used for industrial applications [49]
REFERENCES
- UAE, Water - The Official Portal of the UAE Government,ae, Mar. 11, 2021. https://u.ae/en/information-and-services/environment-and-energy/water-and-energy/wat er-
- Jones, M. Qadir, M. T. H. van Vliet, V. Smakhtin, and S. Kang, The state of desalinationandbrineproduction:Aglobaloutlook,ScienceofTheTotalEnvironment, vol. 657, no. 657, pp. 13431356, Mar. 2019, doi:
https://doi.org/10.1016/j.scitotenv.2018.12.076
- Al-Saidi, A.-K. Ellermann, Markus Frederic Fittkow, Tobias Romanus Perillieux, ImenSaadaoui,andRadhouaneBen-Hamadou,Theperilsofbuildingbig:Desalination sustainability and brine regulation in the Arab Gulf countries,Water Resources and Industry, vol. 32, pp. 100259100259, Jun. 2024, doi: https://doi.org/10.1016/j.wri.2024.100259
- D. Birkett, "Factors Influencing the Economics of Desalination." in
Non-ConventionalWaterResourcesUseinDevelopingCountries.NewYork,United Nations, pp. 89-102. (Natural Resources/Water Series No. 22)
- Behnam,M.Faegh,andM.Khiadani,Areviewonstate-of-the-artapplicationsof data-driven methods in desalination systems,Desalination, vol. 532, p. 115744, Jun. 2022, doi:https://doi.org/10.1016/j.desal.2022.115744.
- Asif, Fundamentals and Application of Solar Thermal Technologies,
ScienceDirect, Jan. 01, 2017.
https://www.sciencedirect.com/science/article/abs/pii/B9780124095489100934
- E. Kabeel, Z. M. Omara, and M. M. Younes, Techniques used to improve the performanceofthesteppedsolarstillAreview,RenewableandSustainableEnergy Reviews, vol. 46, pp. 178188, Jun. 2015, doi:https://doi.org/10.1016/j.rser.2015.02.053.
- Zheng, Solar Energy Desalination Technology,ScienceDirect, 2017.https://www.sciencedirect.com/book/9780128054116/solar-energy-desalination-technology (accessed Nov. 27, 2024).
- LienhardV,Humidification-dehumidificationdesalination,inDesalination:Water from Water, 2nd edition, Chpt.9. Hoboken, NJ: Wiley-Scrivener, 2019. ISBN
978-1-119-40774-4
- Rahimi-Ahar,M.S.Hatamipour,andL.R.Ahar,Air
humidification-dehumidificationprocessfordesalination:Areview,ProgressinEnergy and Combustion Science, vol. 80, p. 100850, Sep. 2020, doi:https://doi.org/10.1016/j.pecs.2020.100850.
- T.Arenaetal.,Managementanddewateringofbrinesextractedfromgeologic carbon storage sites,International Journal of Greenhouse Gas Control, vol. 63, pp. 194214, Aug. 2017, doi: https://doi.org/10.1016/j.ijggc.2017.03.032.
- Ma, C. Zhai, and F. Yu, Review of flow electrode capacitive deionization technology:Researchprogressandfuturechallenges,Desalination,vol.564,pp. 116701116701, Oct. 2023, doi:https://doi.org/10.1016/j.desal.2023.116701.
- Peate and L. Garca-Rodrguez, Current trends and future prospects in the designofseawaterreverseosmosisdesalinationtechnology,Desalination,vol.284,
- 18, Jan. 2012, doi: https://doi.org/10.1016/j.desal.2011.09.010.
- Parani and O. S. Oluwafemi, Membrane Distillation: Recent Configurations, MembraneSurfaceEngineering,andApplications,Membranes,vol.11,no.12,p.934, Nov. 2021, doi:https://doi.org/10.3390/membranes11120934.
- SODIMATE,TheDifferentDesalinationMethods,Sodimate,22,2021.https://sodimate.com/the-different-desalination-methods/
- Al-Amshawee,M.Y.B.M.Yunus,A.A.M.Azoddein,D.G.Hassell,I.H.Dakhil, and H. A. Hasan, Electrodialysis desalination for water and wastewater: A review,Chemical Engineering Journal, vol. 380, p. 122231, Jan. 2020, doi:https://doi.org/10.1016/j.cej.2019.122231
- Pilat, Practice of water desalination by electrodialysis,Desalination, vol. 139, no.13,pp.385392,Sep.2001,doi:https://doi.org/10.1016/s0011-9164(01)00338-1
- Rabiee,K.R.Khalilpour,J.M.Betts,andN.Tapper,Chapter13-Energy-Water Nexus: Renewable-Integrated Hybridized Desalination Systems,ScienceDirect, Jan. 01, 2019.
https://www.sciencedirect.com/science/article/abs/pii/B9780128133064000136
- Riedewald,UTILITIESANDEFFLUENTTREATMENT|WaterSupply,
Academic Press, 2011, pp. 582588. doi:https://doi.org/10.1016/B978-0-12-374407-4.00471-4
- A. Ahmed, S. Amin, and A. A. Mohamed, Fouling in reverse osmosis membranes:monitoring,characterization,mitigationstrategiesandfuturedirections,Heliyon, vol. 9, no. 4, p. e14908, Mar. 2023, doi:https://doi.org/10.1016/j.heliyon.2023.e14908
- S.Rizkand A.S.Alsharhan, Waterresourcesin theUnitedArab Emirates,
ScienceDirect, Jan. 01, 2003, doi:
https://www.sciencedirect.com/science/article/abs/pii/S0167564803800229
- Nautiyal,Varun,andA.Kumar,Reverserunningpumpsanalytical,experimental and computational study: A review, Renewable and Sustainable Energy Reviews, vol. 14, no. 7, pp. 20592067, Sep. 2010, doi: https://doi.org/10.1016/j.rser.2010.04.006
- Hilal, G. J. Kim, and C. Somerfield, Boron removal from saline water: A comprehensivereview,Desalination,vol.273,no.1,pp.2335,Jun.2011,doi:https://doi.org/10.1016/j.desal.2010.05.012.
- BrookandM.A.Dawoud,COASTALWATERRESOURCESMANAGEMENT IN THE UNITED ARAB EMIRATES.
https://www.researchgate.net/publication/238796606_COASTAL_WATER_RESOURCES_MANAGEMENT_IN_THE_UNITED_ARAB_EMIRATES
- A.Sanza,V.Bonnlyea,andG.Cremerb,Fujairahreverseosmosisplant:2 years of operation,Desalination, vol. 203, no. 13, pp. 9199, Feb. 2007, doi:https://doi.org/10.1016/j.desal.2006.03.526
- Ahmed, Brine Dispsoal from Inland Desalination Plants: Current Status, Problems, and Opportunities., Jan. 01, 2004. Doi:https://www.researchgate.net/publication/250002434_Brine_Dispsoal_from_Inland_Desalination_Plants_Current_Status_Problems_and_Opportunities
- BabakZolghadr?Asliet,Areviewoflimitationsandpotentialsofdesalinationas a sustainable source of water,Environmental Science and Pollution Research, Nov. 2023, doi: https://doi.org/10.1007/s11356-023-30662-x
- V.AmmaandF.Ashraf,BrineManagementinReverseOsmosisDesalination:A UAEPerspective,2020AdvancesinScienceandEngineeringTechnologyInternational Conferences (ASET), Feb. 2020, doi: https://doi.org/10.1109/aset48392.2020.9118335.
- C. Chowet al., Numerical Prediction of Background Buildup of Salinity Due to DesalinationBrineDischargesintotheNorthernArabianGulf,Water,vol.11,no.11,p. 2284, Oct. 2019, doi:https://doi.org/10.3390/w11112284.
- Omerspahic, H. Al-Jabri, S. A. Siddiqui, and I. Saadaoui, Characteristics of DesalinationBrineandItsImpactsonMarineChemistryandHealth,WithEmphasison
thePersian/ArabianGulf:AReview,FrontiersinMarineScience,vol.9,Apr.2022,doi: https://doi.org/10.3389/fmars.2022.845113.
- Frank,E.Rahav,andE.Bar-Zeev,Short-termeffectsofSWROdesalination brine on benthic heterotrophic microbial communities, Desalination, vol. 417, pp. 5259, Sep. 2017, doi: https://doi.org/10.1016/j.desal.2017.04.031.
- Sirotaetal.,ImpactsofDesalinationBrineDischargeonBenthicEcosystems,Environmental science & technology, vol. 58, no. 13, pp. 56315645, Mar. 2024, doi: https://doi.org/10.1021/acs.est.3c07748.
- L.Cambridge,A.Zavala-Perez,G.R.Cawthray,J.Statton,J.Mondon,andG.
- Kendrick,Effectsofdesalinationbrineandseawaterwiththesameelevatedsalinity on growth, physiology and seedling development of the seagrass Posidonia australis,Marine Pollution Bulletin, vol. 140, pp. 462471, Mar. 2019, doi:https://doi.org/10.1016/j.marpolbul.2019.02.001.
- Capet al., Hypersaline water from desalinization plants causes oxidative damageinPosidoniaoceanicameadows,ScienceofTheTotalEnvironment,vol.736,
- 139601, May 2020, doi:https://doi.org/10.1016/j.scitotenv.2020.139601.
[35]Y.Fernndez-Torquemada,A.Carratal,andJ.L.Snchez-Lizaso,Impactofbrine on the marine environment and how it can be reduced,rua.ua.es, Nov. 2019, doi:https://doi.org/10.5004/dwt.2019.24615.
- Ma, C. Zhai, and F. Yu, Review of flow electrode capacitive deionization technology:Researchprogressandfuturechallenges,Desalination,vol.564,pp. 116701116701, Oct. 2023, doi: https://doi.org/10.1016/j.desal.2023.116701.
- ElsaidK,KamilM,SayedET,AbdelkareemMA,WilberforceT,Olabi
Environmentalimpactofdesalinationtechnologies:Areview.SciTotalEnviron.2020
Dec15;748:141528.doi:10.1016/j.scitotenv.2020.141528.Epub2020Aug8.PMID:
32818886.
- Guanetal.,ComparativeGenomicsoftheGenusMethanohalophilus,Including a Newly Isolated Strain From Kebrit Deep in the Red Sea,Frontiers in Microbiology, vol. 10, Apr. 2019, doi: https://doi.org/10.3389/fmicb.2019.00839.
- R.SowersandJ.G.Ferry,IsolationandCharacterizationofaMethylotrophic MarineMethanogen,Methanococcoidesmethylutensgen.nov.,sp.nov,Appliedand Environmental Microbiology, vol. 45, no. 2, pp. 684690, 1983, doi: https://doi.org/10.1128/aem.45.2.684-690.1983.
- Singh,M.M.Kendall,Y.Liu,andD.R.Boone,Isolationandcharacterizationof methylotrophic methanogens from anoxic marine sediments in Skan Bay, Alaska: description of Methanococcoides alaskense sp. nov., and amended description of Methanosarcina baltica,International Journal of Systematic and Evolutionary Microbiology, vol. 55, no. 6, pp. 25312538, Nov. 2005, doi: https://doi.org/10.1099/ijs.0.63886-0.
- Yinetal.,CO2conversiontomethaneandbiomassinobligatemethylotrophic methanogensinmarinesediments,TheISMEJournal,vol.13,no.8,pp.21072119, Apr. 2019, doi:https://doi.org/10.1038/s41396-019-0425-9.
- P. Bueno de Mesquita, D. Wu, and S. G. Tringe, Methyl-Based Methanogenesis:anEcologicalandGenomicReview,Microbiologyandmolecular biology reviews: MMBR, vol. 87, no. 1, p. e0002422, Mar. 2023, doi: https://doi.org/10.1128/mmbr.00024-22.
- Liang et al., Unraveling methanogenesis processes and pathways for Quaternaryshallowbiogenicgasinaquifersystemsthroughgeochemical,genomicand transcriptomic analyses,Science of The Total Environment, vol. 955, p. 177189, Oct. 2024, doi: https://doi.org/10.1016/j.scitotenv.2024.177189.
- A.Richards,T.J.Lie,J.Zhang,S.W.Ragsdale,J.A.Leigh,andN.D.Price, Exploring Hydrogenotrophic Methanogenesis: a Genome Scale Metabolic ReconstructionofMethanococcusmaripaludis,JournalofBacteriology,vol.198,no. 24, pp. 33793390, Oct. 2016, doi: https://doi.org/10.1128/jb.00571-16.
- Krich, D. Augenstein, J. P. Batmale, J. Benemann, B. Rutledge, and D. Salour, BiomethanefromDairyWaste,aSourcebookfortheProductionandUseofRenewable Natural Gas,escholarship.org, Jul. 2005, Available: https://escholarship.org/uc/item/35k1861z
- Usman, L. T. Yogarathinam, N. Baig, S. I. Abba, R. Chrystie, and I. H. Aljundi, MXene-enhancedsulfonatedTFNnanofiltrationmembranesforimproveddesalination performance,Desalination, vol. 581, p. 117566, Mar. 2024, doi: https://doi.org/10.1016/j.desal.2024.117566.
- Ramato Ashu Tufa, Gianluca Di Profio, Enrica Fontananova, A. H. Avci, and E. Curcio,ForwardOsmosis,ReverseElectrodialysisandMembraneDistillation,Elsevier eBooks, pp. 365385, Jan. 2019, doi:
https://doi.org/10.1016/b978-0-12-813551-8.00015-2.
- Boo,R.K.Winton,K.M.Conway,andN.Y.Yip,Membrane-lessand
Non-Evaporative Desalination of Hypersaline Brines by Temperature Swing Solvent Extraction,EnvironmentalScience&TechnologyLetters,vol.6,no.6,pp.359364, Apr. 2019, doi: https://doi.org/10.1021/acs.estlett.9b00182.
- ElsayedandA.H.Al-Marzouqi,Pathtowardsustainabledesalination:Sodium precipitation and carbon capture,Desalination, vol. 549, p. 116324, Mar. 2023, doi: https://doi.org/10.1016/j.desal.2022.116324.
GLOSSARY
- Methanosarcinaceae :A taxonomic group that comes under the order Methanosarcinales. Theircharacteristicfeatureisthattheyaremethanogensthat incorporate the unusual amino acid pyrrolysine into their enzymes.
- Methanogenesis :Methanogenesis is Methanogenesis is the process of oxidation of biodegradation of organic rich substrates mediated by various microbial population that functions under syntrophic anaerobic conditions
- Photovoltaic panel :A photovoltaic panel(solarpanel)isadevicethatconverts sunlight into electricity by using photovoltaic (PV) cells. PV cells are made of materials that produce excited electrons when exposed to light.
- Anti-fouling agents :Antifouling agents refers to chemicals that are used to prevent the attachment of undesired fouling substances on the RO membrane that reduce membrane efficiency by clogging pores.
- Effluent :Effluent refers to the clean filtrate produced after any water treatment process
- Bathymetry :The measurement of depth of water inoceans,seas, or
- Echinoderms :An echinoderm is any animal of thephylumEchinodermata, which includes starfish, brittlestars, sea urchins, sand dollars and sea cucumbers, as well as the sessile sea lilies or "stone lilies".
- Biocides :Biocides aresubstancesintendedtodestroy,deter,renderharmless, prevent the action of, or otherwise exert a controlling effect on any harmful organism by chemical or biological means
- Bio-indicator :Bioindicators are living organisms such as plants, planktons, animals, and microbes, which are utilized to screen the health of the natural ecosystem in the environment
- Calcareous :Calcareous is an adjective meaning "mostly or partly composed of calcium carbonate
- Necrosis :Necrosis refers to the death of body tissue due to cell death
- Acetoclastic :Production of methane from acetic acid and other
- Mixitrophically :Ability to interchange between various metabolic pathways of methanogenesis with change in substrate concentrations and environmental

