To what extent does a change in the ionic radius of the anion in salts affect enthalpy of solution ?
- Country :
Australia
1.Rationale
Enthalpy is defined as a thermodynamic quantity equivalent to the total heat content of a system. (Oxford, 2023). When conducting an experiment, the calculated enthalpy is important as it allows scientists to determine the amount of energy that is within a system. Dissolution is the process where a solute in gaseous, liquid, or solid phase dissolves in a solvent to form a solution (Lu, 2022). The lattice energy or enthalpy of an ionic compound is defined as the energy required to separate one mole of the solid into its component gaseous ions (LibreTexts, 2020). The lattice energy of an ionic crystal can be expressed by the following equation (derived from Coulombs law, governing the forces between electric charges).
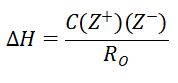
Where C is the specific heat capacity, Z+ and Z- are the charges of the ions and RO is the interionic distance. During a dissolution reaction, a reactant substance is transformed into a product substance. To find the overall enthalpy of solution, the hydration enthalpy, which is the energy required to make new bonds, subtracted from the lattice enthalpy. This new substance may have a di?erent amount of chemical energy stored in its bonds, so the reaction will either absorb or release energy as it progresses from reactants to products. If energy is released the temperature increases and is therefore an exothermic reaction and when energy is absorbed the temperature decreases in an endothermic reaction (Pearson, 2019). The original experiment measured the temperature change and hence the enthalpy of three different reactions in a calorimeter. The technique of calorimetry is used to measure amounts of heat transferred to or from a substance (OpenStaxCollege, 2014).
The results from the original experiment provided valuable data in order to formulate a rational hypothesis for the modified experiment. The original experiment possessed some limitations such as each substance used only provided results in terms of the enthalpy of that single reaction as well as using tap water instead of deionised water, so the substances would be dissolved in pure water.
The modified experiment aimed measure enthalpy of three salts with a varied anion (NaCl, NaBr, NaI). As ionic radius increases, lattice enthalpy decreases and therefore enthalpy of solution decreases (Pearson, 2019). By performing 3 trials with a constant cation (Na) and increasing the ionic radius of the anion (Cl, Br, I) it is hypothesised that the enthalpy of solution will decrease as the ionic radius of anion increases in correlation to coulombs law.
2.Research Question
To what extent does a change in the ionic radius of the anion in salts (NaCl, NaBr, NaI) affect enthalpy of solution where the cation (Na) is kept constant?
3.Methodology
Original Experiment small paragraph + intro to modified
The original experiment used three different reactants and dissolved them in three different solvents. The temperature change was recorded from these reactions with a glass thermometer and the enthalpy was then calculated. The results from the original experiment gave valuable data and insight between the relationship of temperature and enthalpy. The experiment whilst it provided valuable data, however possessed some limitations and therefore impacted the validity of the data.
Modifications
Modifications were made to the original experiment in order to investigate the affect ionic radius has on enthalpy. Instead of using 3 different solutes and solvents, 3 similar salts with a cation of increasing ionic radius were dissolved in a solvent that was distilled water. By having the same solvent and similar solutes, there is more room for investigation as the experiment no longer lacks minor difference in order for more precise data to be obtained. The original experiment was also refined and extended in various in order to continue to perform fair trials and to increase the reliability of the data.
Extended by:
In this experiment, the use of three trials allows for the calculation of an average value, increases the accuracy and precision of the measurements taken. Additionally, the identification of any outliers in the data can help to detect any potential errors or inconsistencies in the experimental procedure. Furthermore, the use of three similar salts (NaCl, NaBr, NaI) with increasing ionic radius can provide insight into the effect of ionic size on the enthalpy of the solution. By comparing the results obtained for each salt, it may be possible to identify any trends or patterns that exist in the data. Finally, assessing the reliability of the experimental results is critical to ensuring the validity of the conclusions drawn from the study. By examining the data for consistency and reproducibility, there will be greater confidence in the accuracy of findings.
Refined by:
Temperature probe -
By using a temperature probe, the temperature readings obtained were more accurate and precise, as the probe could measure temperatures to a greater number of significant figures than a traditional thermometer. This refinement ensured that the results obtained were more reliable, as measurement errors due to temperature variations were significantly reduced.
Stirrer bar -
Another refinement that was introduced was the use of a stirrer bar. By incorporating a stirrer bar, a consistent RPM was maintained in each trial, which enhanced the reproducibility of the experiment. This refinement ensured that the results obtained were more reliable, as it reduced the likelihood of variations in temperature due to differences in stirring speed.
3g of salt each trial -
In addition to the above, a consistent amount of 3g of salt was added in each trial. The logic behind this refinement was to obtain consistent changes in temperature, which would allow for better comparison of results and obtaining a more reliable average. By adding a consistent amount of salt in each trial, the variability in results was minimized, ensuring that the results obtained were more reliable and accurate.
Measuring cylinder to measure water
To minimize errors in the calculation of the heat of solution, a measuring cylinder was used to measure a known volume of water. This refinement enhanced the accuracy of the calculation, as the volume of water used in each trial was consistent and known. This ensured that the results obtained were more reliable, as measurement errors due to variations in the volume of water used were significantly reduced.
Deionized/Distilled water -
Finally, deionized, or distilled water was used to avoid any salts already dissolved in the water. This refinement ensured that the experiment was free from any external influences that could interfere with the experimental results. By using deionized or distilled water, the results obtained were more reliable and accurate, as the experiment was not influenced by any external factors.
Management of risks
For the most part, sodium chloride, bromide and iodide arent health hazards, but in excessive amounts they can irritate the: eyes. skin. and airways (Fischer Scientific, 2018). In order to decrease the risk of damage or injury occurring, eye protection (goggles) will be worn as well as gloves and aprons. Any solution that touches the skin will be washed off immediately. All waste materials will be disposed of in a large waste beaker and then will be returned to the prep room.
Processing and Analysis of Data
During the dissolution process, the rate of temperature changes only increased or decreased in miniscule increments. These rates of temperature change were maximised by the magnetic stirrer bar operating at a high rpm in order to spreads the solvent's molecules around the solute and therefore increase the chances of collision.
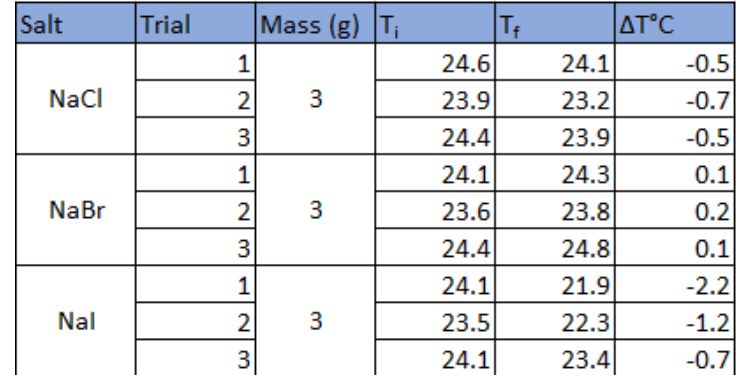
4.1 Raw Data
4.2 Processing of Data
Raw data seen in table 1 was processed using multiple mathematical formulas to determine the change in temperature from each trial in order to eventually calculate the enthalpy of the solution. Table 2 shows the various formulas used to the process the data along with a sample calculation taken from the first trial of the sodium chloride reaction.
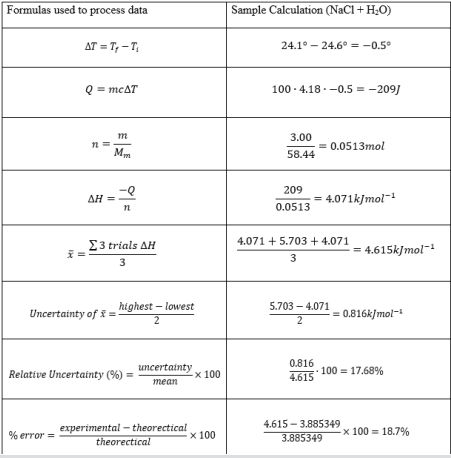
The formulas seen in table 2, were then utilised to create a processed data table (Table 3) that included the mean enthalpy, uncertainty and percentage error. Using literature values for the theoretical enthalpy value, units was converted from cal/mole to kJ/mole. This allowed for percentage error to be calculated and provide insight into the accuracy of the experimental results compared the theoretical values. A mean enthalpy was calculated for each of the three salts in order to investigate the effect ionic radius has on enthalpy.
The data in Table 3 and Graph 1 provides clear evidence of the relationship between ionic radius and enthalpy. As ionic radius increases, there is a decrease in enthalpy, which suggests that larger ions have weaker intermolecular forces with the solvent molecules, resulting in a less exothermic reaction when the salt is dissolved. The data point for sodium iodide, which has a much higher enthalpy value than expected, could be due to experimental errors or systematic errors in the measurement process. This indicates that further investigations need to be carried out to improve the experimental procedure.
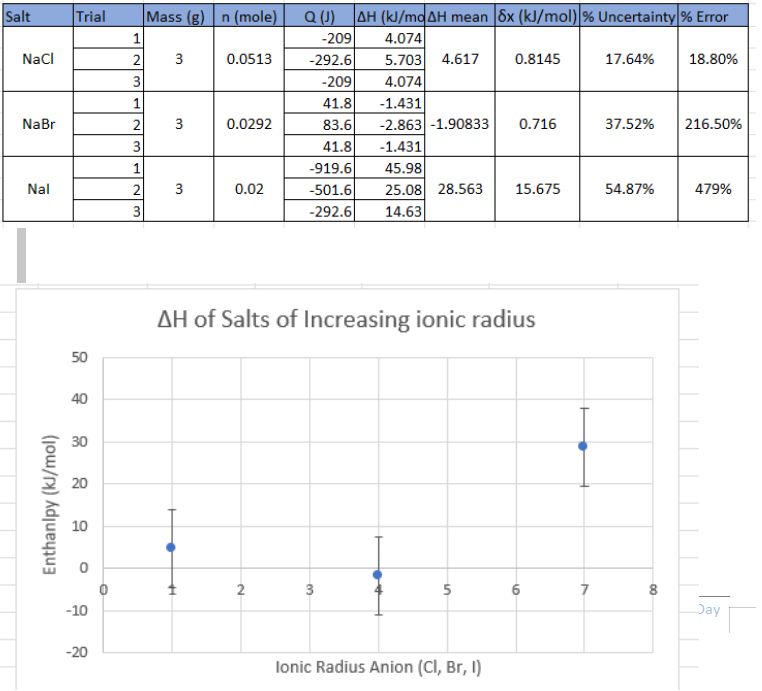
The high percentage error given from two of the salts indicate the accuracy of the experiment. With a high percentage error, it shows how far off the experimental values were to what the values theoretically should be. The experiment however was precise in terms of the values of the trials. This can be seen by the percentage uncertainty of the 3 salts.
If the theoretical value for sodium iodide was used there would a be linear negative trend indicating that enthalpy of solution decreases as ionic radius increases. Therefore, the experimental value for sodium iodide suggests that was an error in measuring the original temperature change or perhaps error with equipment used.
Overall, this experiment provides valuable insight into the relationship between ionic radius and enthalpy. The data suggests that increasing ionic radius results in a decrease in enthalpy, indicating weaker intermolecular forces between the ions and solvent molecules. However, the high percentage error for sodium iodide suggests that further investigations are necessary to confirm this trend.
Evaluation
The experiment investigated the relationship between ionic radius and enthalpy during the dissolution process of three salts: sodium chloride, potassium chloride, and sodium iodide. The raw data was processed using mathematical formulas to calculate the change in temperature for each trial, and the enthalpy of solution was then calculated.
One strength of the experiment is the use of multiple trials for each salt, which increased the reliability of the results by reducing the impact of random error. The use of mathematical formulas to process the data can also increase precision by providing a standardized method for calculating the enthalpy of solution.
However, there are weaknesses in the experiment. The high percentage error for two of the salts suggests that there may have been accuracy issues with the experiment, which could be due to factors such as measurement error or systematic error. The potential impact of human error, such as incorrect calculations or misreading measurements, is also a concern. In terms of reliability and validity, the precision of the data calculated impacts the experiments overall reliability while the low accuracy of the enthalpies for two of the salts which were not close to the theoretical values as well as the large error bars indicating the lack of validity.
In summary, while the experiment provides valuable insights into the relationship between ionic radius and enthalpy, there are weaknesses in the accuracy and control of variables that should be addressed refined next time to improve the reliability and validity of the results.

